The atmosphere slowly oxidizes hydrocarbons in a number of steps that eventually convert the hydrocarbon into carbon di- oxide and water. The overall reaction of a number of such steps for methane gas is CHĄ(g) + 5 02(g) + 5 NO(g) CO(8) + H,O(g) + 5 NO-(g) + 2 OH(g) Suppose that an atmospheric chemist combines 155 ml of methane at STP, 885 mL of oxygen at STP, and 55.5 mL of NO at STP in a 2.0 L flask. The flask is allowed to stand for several weeks at 275 K. If the reaction reaches 90.0% of completion (90.0% of the limiting reactant is consumed), what is the partial pressure of each of the reactants and products in the flask at 275 K? What is the total pressure in the flask?
The atmosphere slowly oxidizes hydrocarbons in a number of steps that eventually convert the hydrocarbon into carbon di- oxide and water. The overall reaction of a number of such steps for methane gas is CHĄ(g) + 5 02(g) + 5 NO(g) CO(8) + H,O(g) + 5 NO-(g) + 2 OH(g) Suppose that an atmospheric chemist combines 155 ml of methane at STP, 885 mL of oxygen at STP, and 55.5 mL of NO at STP in a 2.0 L flask. The flask is allowed to stand for several weeks at 275 K. If the reaction reaches 90.0% of completion (90.0% of the limiting reactant is consumed), what is the partial pressure of each of the reactants and products in the flask at 275 K? What is the total pressure in the flask?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.61PAE: 61 As one step in its purification, nickel metal reacts with carbon monoxide to form a compound...
Related questions
Question
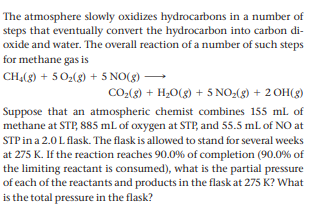
Transcribed Image Text:The atmosphere slowly oxidizes hydrocarbons in a number of
steps that eventually convert the hydrocarbon into carbon di-
oxide and water. The overall reaction of a number of such steps
for methane gas is
CHĄ(g) + 5 02(g) + 5 NO(g)
CO(8) + H,O(g) + 5 NO-(g) + 2 OH(g)
Suppose that an atmospheric chemist combines 155 ml of
methane at STP, 885 mL of oxygen at STP, and 55.5 mL of NO at
STP in a 2.0 L flask. The flask is allowed to stand for several weeks
at 275 K. If the reaction reaches 90.0% of completion (90.0% of
the limiting reactant is consumed), what is the partial pressure
of each of the reactants and products in the flask at 275 K? What
is the total pressure in the flask?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning