The circles in the diagrams below represent energy levels in an atom, and the arrows show electron transitions from one energy level to another. The spacing between circles is proportional to the differences in energy. Rank the atoms based on the energy of the photon that is absorbed, from lowest to highest. ONLY rank those transitions that absorb a photon and that are physically possible. A) D)
- The circles in the diagrams below represent energy levels in an atom, and the arrows show electron transitions from one energy level to another. The spacing between circles is proportional to the differences in energy. Rank the atoms based on the energy of the photon that is absorbed, from lowest to highest. ONLY rank those transitions that absorb a photon and that are physically possible. (A,B,C,D,E,F)
Hint: It is not possible for an electron to begin or end in the nucleus.
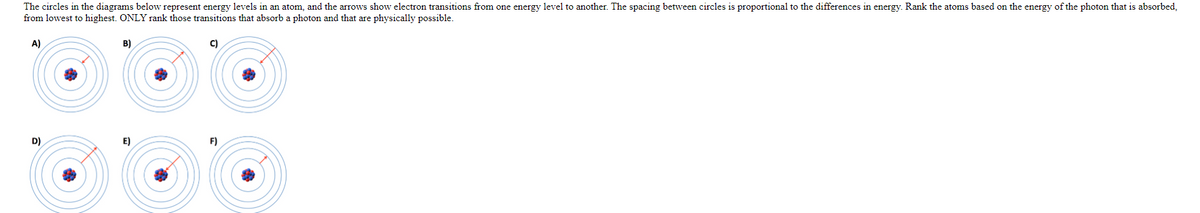
In an atom, the electrons are in constant motion around the specific nucleus in specific orbits or energy level. It is possible that electrons jump from lower energy to higher energy or in some cases higher energy to lower energy.
The lowest energy level of the electron is the ground state while the higher levels are the excited state.
When an atom jumps from the lower energy level to higher energy level, it requires energy. Therefore, the energy required for such transitions are provided by absorption of photon.
When an atom moves from the higher energy level to lower energy level, it releases the excess energy as photon emission.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps









