Part A In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital. A single box represents an orbital. The unpaired electron is represented as 1 whereas the paired electrons in the same orbital ar represented by two arrows pointing in opposite directions: 11. Watch the animation and identify which of the following statements are correct. Check all that apply. > View Available Hint(s) O In the Li atom, the 3s. 3p, and 3d orbitals have different energies
Part A In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital. A single box represents an orbital. The unpaired electron is represented as 1 whereas the paired electrons in the same orbital ar represented by two arrows pointing in opposite directions: 11. Watch the animation and identify which of the following statements are correct. Check all that apply. > View Available Hint(s) O In the Li atom, the 3s. 3p, and 3d orbitals have different energies
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 105E: One bit of evidence that the quantum mechanical model is correct lies in the magnetic properties of...
Related questions
Question
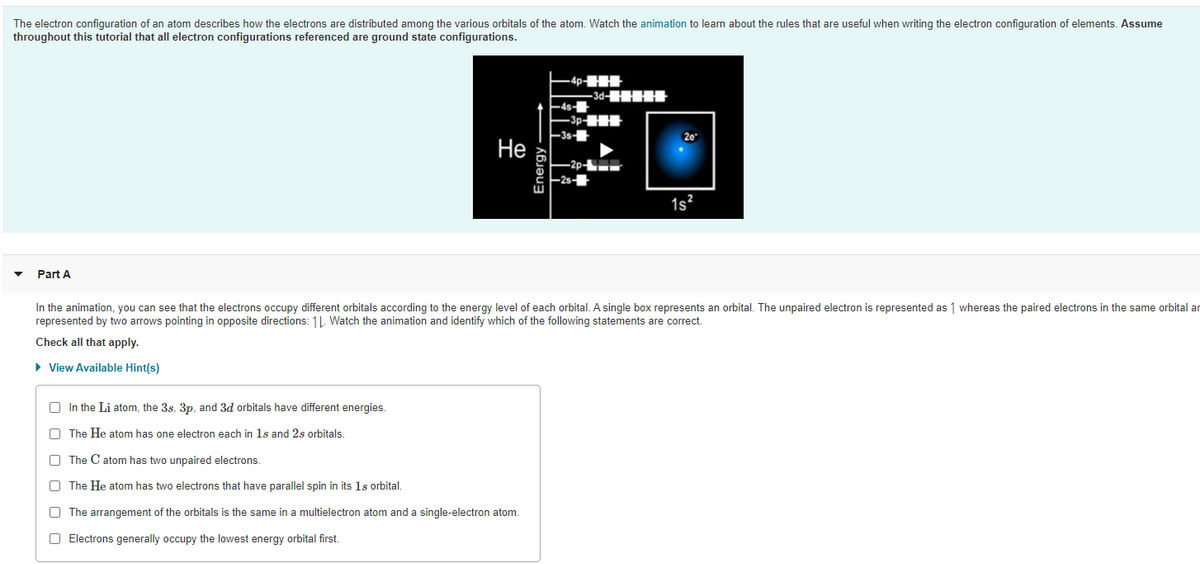
Transcribed Image Text:The electron configuration of an atom describes how the electrons are distributed among the various orbitals of the atom. Watch the animation to learn about the rules that are useful when writing the electron configuration of elements. Assume
throughout this tutorial that all electron configurations referenced are ground state configurations.
-4pH
–3d-HH
-4s-
-3p +
-3s-
2e
Не
1s?
Part A
In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital. A single box represents an orbital. The unpaired electron is represented as 1 whereas the paired electrons in the same orbital ar
represented by two arrows pointing in opposite directions: 11. Watch the animation and identify which of the following statements are correct.
Check all that apply.
• View Available Hint(s)
O In the Li atom, the 3s, 3p, and 3d orbitals have different energies.
O The He atom has one electron each in ls and 2s orbitals.
The C atom has two unpaired electrons.
O The He atom has two electrons that have parallel spin in its 1s orbital.
O The arrangement of the orbitals is the same in a multielectron atom and a single-electron atom.
O Electrons generally occupy the lowest energy orbital first.
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning