The compound below is treated with N-bromosuccinimide (NBS) in t presence of light. Draw both resonance structures for the radical produced by reaction o compound with a bromine atom. Assume reaction occurs only at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. Separate resonance structures using the → symbol from the č down menu. • Include all valence radical electrons in your answer.
The compound below is treated with N-bromosuccinimide (NBS) in t presence of light. Draw both resonance structures for the radical produced by reaction o compound with a bromine atom. Assume reaction occurs only at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. Separate resonance structures using the → symbol from the č down menu. • Include all valence radical electrons in your answer.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter29: Organic Polymer Chemistry
Section: Chapter Questions
Problem 29.28P
Related questions
Question
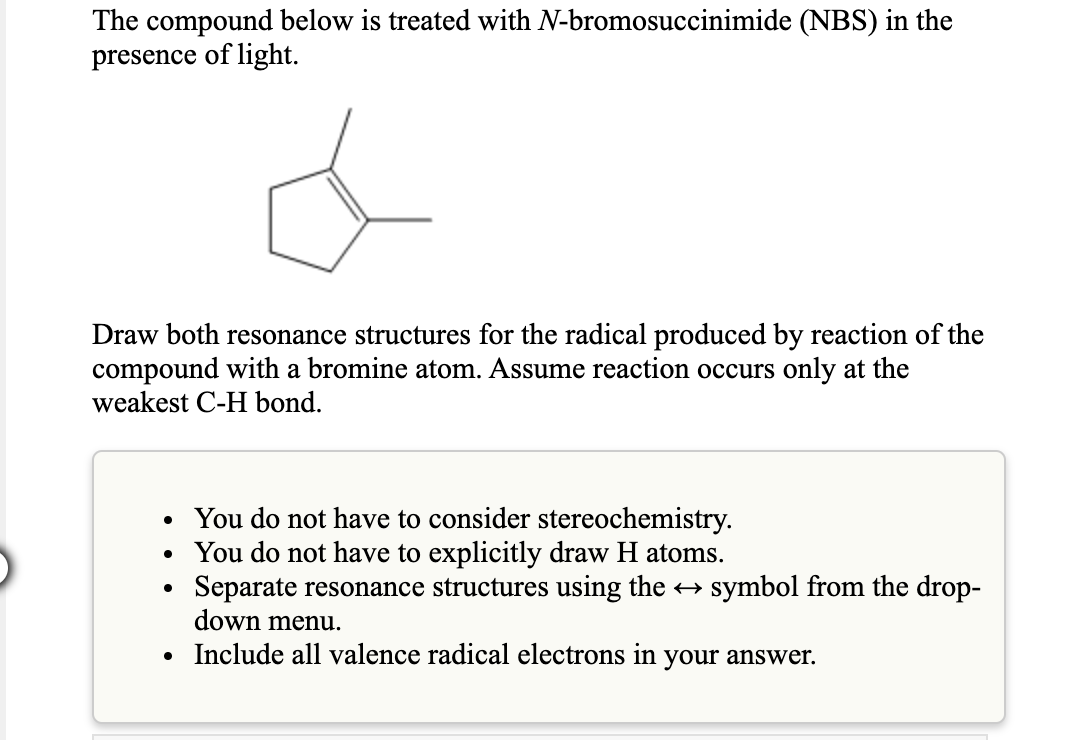
Transcribed Image Text:The compound below is treated with N-bromosuccinimide (NBS) in the
presence of light.
Draw both resonance structures for the radical produced by reaction of the
compound with a bromine atom. Assume reaction occurs only at the
weakest C-H bond.
• You do not have to consider stereochemistry.
You do not have to explicitly draw H atoms.
Separate resonance structures using the symbol from the drop-
down menu.
• Include all valence radical electrons in your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning