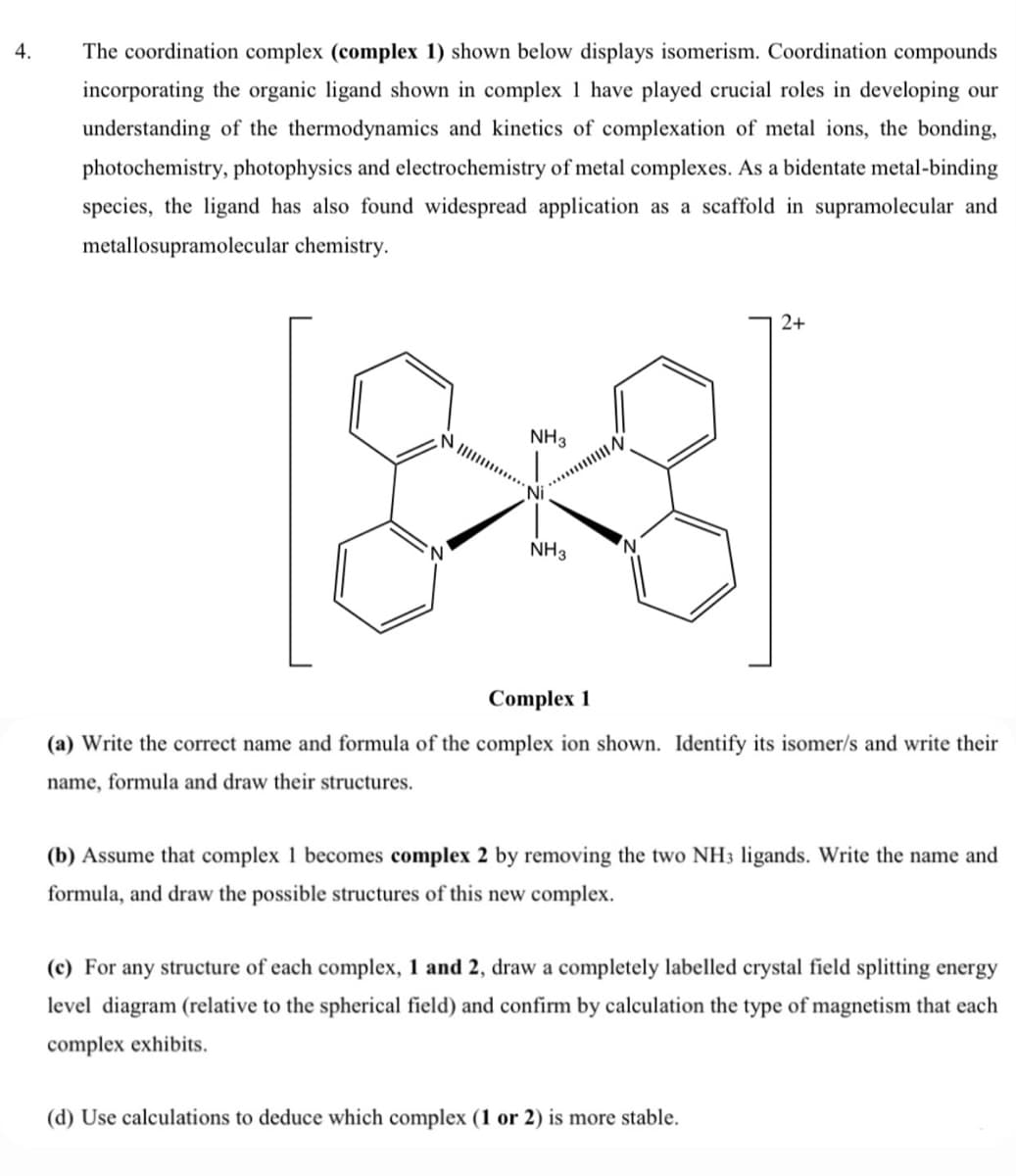
The coordination complex (complex 1) shown below displays isomerism. Coordination compounds incorporating the organic ligand shown in complex 1 have played crucial roles in developing our understanding of the thermodynamics and kinetics of complexation of metal ions, the bonding, photochemistry, photophysics and electrochemistry of metal complexes. As a bidentate metal-binding species, the ligand has also found widespread application as a scaffold in supramolecular and metallosupramolecular chemistry. 2+ NI NH3 NH3 Complex 1 (a) Write the correct name and formula of the complex ion shown. Identify its isomer/s and write their name, formula and draw their structures. (b) Assume that complex 1 becomes complex 2 by removing the two NH3 ligands. Write the name and formula, and draw the possible structures of this new complex. (c) For any structure of each complex, 1 and 2, draw a completely labelled crystal field splitting energy level diagram (relative to the spherical field) and confirm by calculation the type of magnetism that each complex exhibits.
The coordination complex (complex 1) shown below displays isomerism. Coordination compounds incorporating the organic ligand shown in complex 1 have played crucial roles in developing our understanding of the thermodynamics and kinetics of complexation of metal ions, the bonding, photochemistry, photophysics and electrochemistry of metal complexes. As a bidentate metal-binding species, the ligand has also found widespread application as a scaffold in supramolecular and metallosupramolecular chemistry. 2+ NI NH3 NH3 Complex 1 (a) Write the correct name and formula of the complex ion shown. Identify its isomer/s and write their name, formula and draw their structures. (b) Assume that complex 1 becomes complex 2 by removing the two NH3 ligands. Write the name and formula, and draw the possible structures of this new complex. (c) For any structure of each complex, 1 and 2, draw a completely labelled crystal field splitting energy level diagram (relative to the spherical field) and confirm by calculation the type of magnetism that each complex exhibits.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter21: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 108IP: a. In the absorption spectrum of the complex ion Cr(NCS)63, there is a band corresponding to the...
Related questions
Question
Transcribed Image Text:4.
The coordination complex (complex 1) shown below displays isomerism. Coordination compounds
incorporating the organic ligand shown in complex 1 have played crucial roles in developing our
understanding of the thermodynamics and kinetics of complexation of metal ions, the bonding,
photochemistry, photophysics and electrochemistry of metal complexes. As a bidentate metal-binding
species, the ligand has also found widespread application as a scaffold in supramolecular and
metallosupramolecular chemistry.
2+
NIII
NH3
NH3
Complex 1
(a) Write the correct name and formula of the complex ion shown. Identify its isomer/s and write their
name, formula and draw their structures.
(b) Assume that complex 1 becomes complex 2 by removing the two NH3 ligands. Write the name and
formula, and draw the possible structures of this new complex.
(c) For any structure of each complex, 1 and 2, draw a completely labelled crystal field splitting energy
level diagram (relative to the spherical field) and confirm by calculation the type of magnetism that each
complex exhibits.
(d) Use calculations to deduce which complex (1 or 2) is more stable.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning