The effective nuclear charge experienced by the outer most electron of NA is different than the effective nuclear charge experience by the outer most electron of an NE this difference best accounts for which of the following
The effective nuclear charge experienced by the outer most electron of NA is different than the effective nuclear charge experience by the outer most electron of an NE this difference best accounts for which of the following
Chapter3: Atoms And Elements
Section: Chapter Questions
Problem 35E: We have seen that the reactivity of an element is determined by its electron configuration. What is...
Related questions
Question
The effective nuclear charge experienced by the outer most electron of NA is different than the effective nuclear charge experience by the outer most electron of an NE this difference best accounts for which of the following?
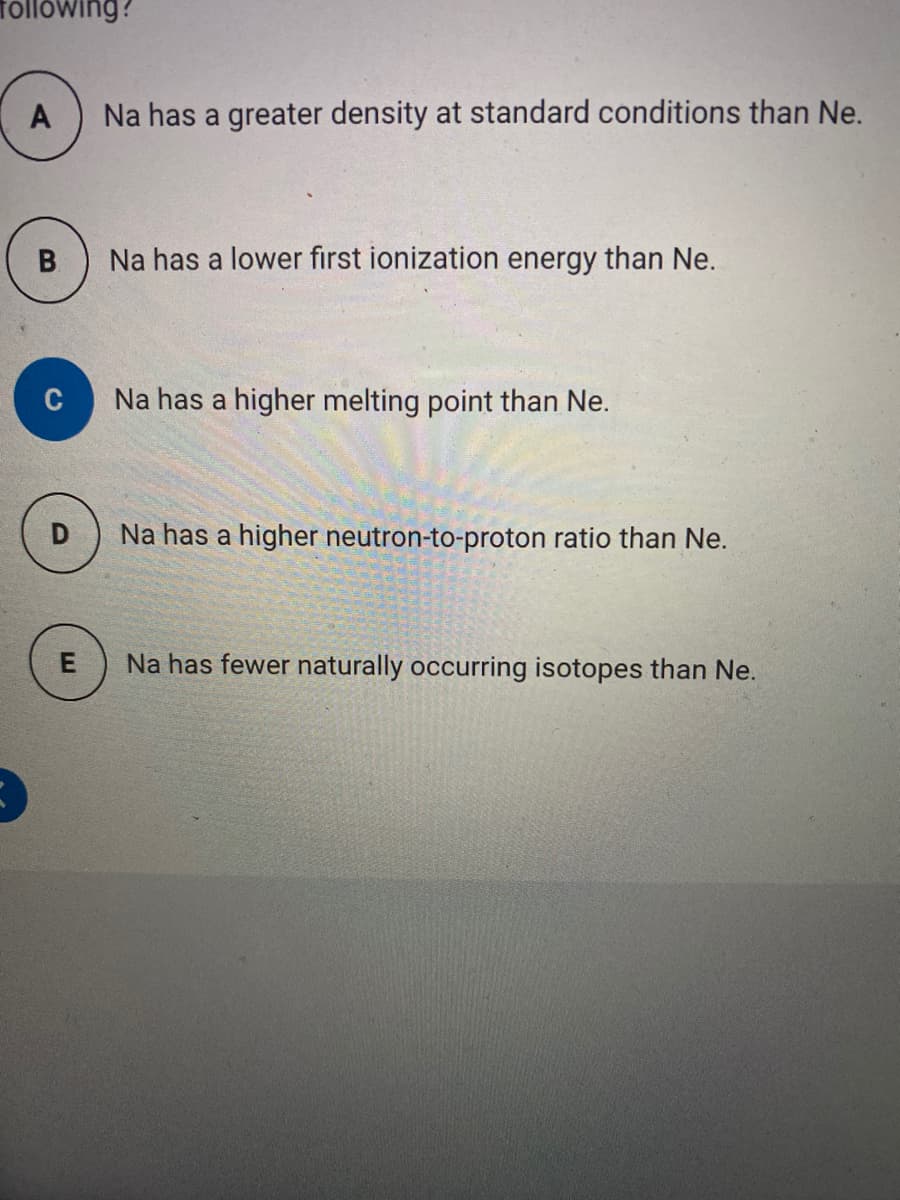
Transcribed Image Text:Tollówing?
A
Na has a greater density at standard conditions than Ne.
B.
Na has a lower first ionization energy than Ne.
C
Na has a higher melting point than Ne.
Na has a higher neutron-to-proton ratio than Ne.
E
Na has fewer naturally occurring isotopes than Ne.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning