The enthalpy change for a chemical reaction isthe sum of the energy consumed in breaking bonds and the energy released during bond formation. One way to determine the overall energy change for a chemical reaction is to apply Hess's law to add together a group of reactions which can be arranged such that the chemical equations, when combined, give the overall equation we are trying to characterize, H. 1st attempt Part 1 See Periodic Table C See Hint Write a balanced chemical equation for the combustion of gaseous propane in gaseous oxygen to produce gaseous carbon dioxide and liquid water. He 5. C;Hg (g) - co.(g) - H,0(g) (3)0 Combine the following equations to determine the enthalpy change for the combustion of 1 mole of propane. Assume that solid carbon is graphite. 3C (s.graphite) +4H, (g) C,H;(g) AHP = -103.8 kJ/mol C(s.graphite) +0,(g) co,(g) AH°= -393.5 kJ/mol H, (2) + 0,(G) H,0(g) AH°= -285.8 kJ/mol kJ/mol Part 3 O See Hint The average propane cylinder for a residential grilli.holds approximatery 18 kg of propane. How much energy (inkJ) is released by the combustion of 12.50kilograms of propane in sufficient Oxygen?
The enthalpy change for a chemical reaction isthe sum of the energy consumed in breaking bonds and the energy released during bond formation. One way to determine the overall energy change for a chemical reaction is to apply Hess's law to add together a group of reactions which can be arranged such that the chemical equations, when combined, give the overall equation we are trying to characterize, H. 1st attempt Part 1 See Periodic Table C See Hint Write a balanced chemical equation for the combustion of gaseous propane in gaseous oxygen to produce gaseous carbon dioxide and liquid water. He 5. C;Hg (g) - co.(g) - H,0(g) (3)0 Combine the following equations to determine the enthalpy change for the combustion of 1 mole of propane. Assume that solid carbon is graphite. 3C (s.graphite) +4H, (g) C,H;(g) AHP = -103.8 kJ/mol C(s.graphite) +0,(g) co,(g) AH°= -393.5 kJ/mol H, (2) + 0,(G) H,0(g) AH°= -285.8 kJ/mol kJ/mol Part 3 O See Hint The average propane cylinder for a residential grilli.holds approximatery 18 kg of propane. How much energy (inkJ) is released by the combustion of 12.50kilograms of propane in sufficient Oxygen?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.103PAE: 9.103 One reason why the energy density of a fuel is important is that to move a vehicle one must...
Related questions
Question
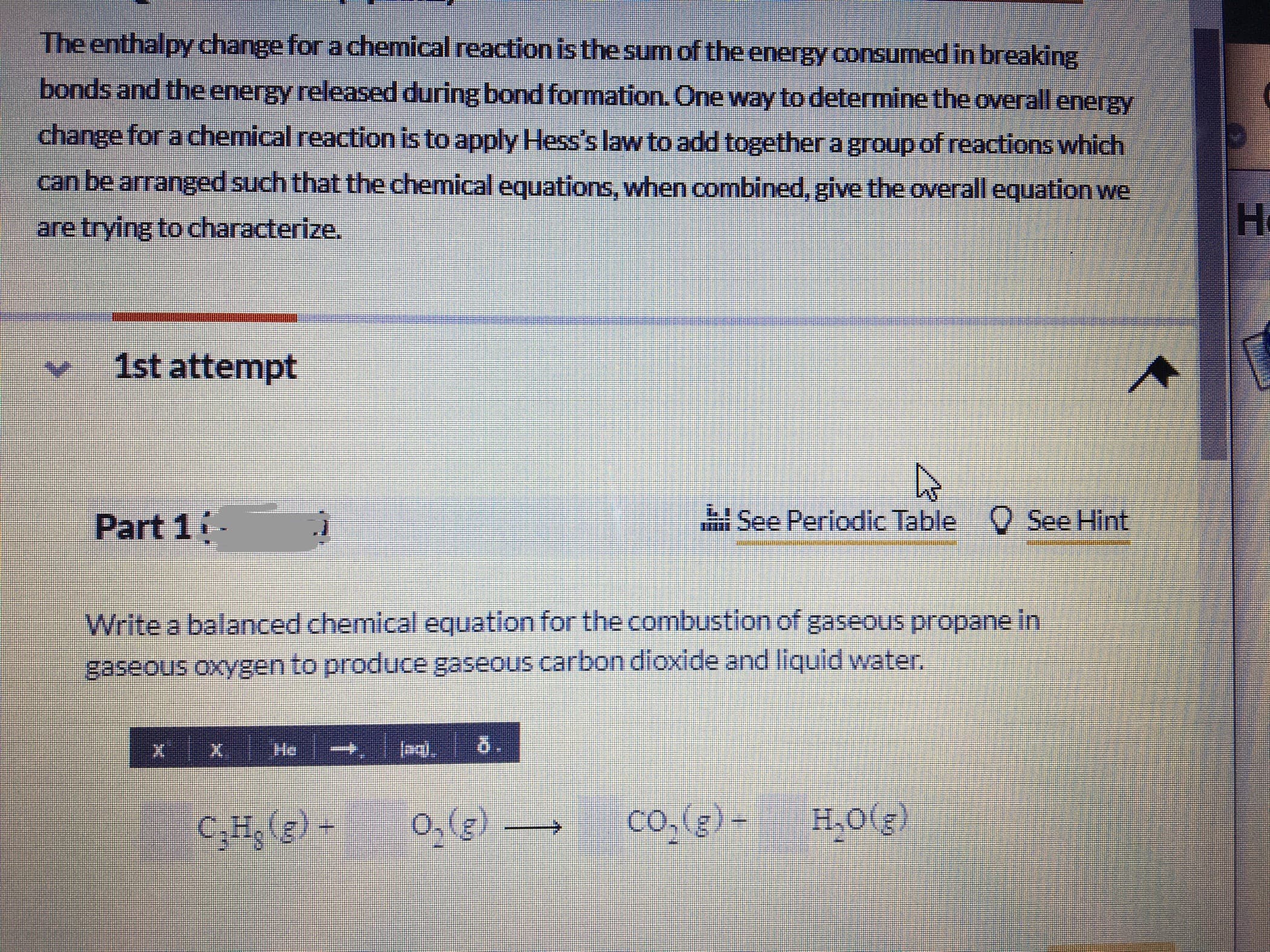
Transcribed Image Text:The enthalpy change for a chemical reaction isthe sum of the energy consumed in breaking
bonds and the energy released during bond formation. One way to determine the overall energy
change for a chemical reaction is to apply Hess's law to add together a group of reactions which
can be arranged such that the chemical equations, when combined, give the overall equation we
are trying to characterize,
H.
1st attempt
Part 1
See Periodic Table C See Hint
Write a balanced chemical equation for the combustion of gaseous propane in
gaseous oxygen to produce gaseous carbon dioxide and liquid water.
He
5.
C;Hg (g) -
co.(g) -
H,0(g)
(3)0

Transcribed Image Text:Combine the following equations to determine the enthalpy change for the combustion of 1 mole of
propane. Assume that solid carbon is graphite.
3C (s.graphite) +4H, (g)
C,H;(g)
AHP = -103.8 kJ/mol
C(s.graphite) +0,(g)
co,(g)
AH°= -393.5 kJ/mol
H, (2) + 0,(G)
H,0(g)
AH°= -285.8 kJ/mol
kJ/mol
Part 3
O See Hint
The average propane cylinder for a residential grilli.holds approximatery 18 kg of propane. How
much energy (inkJ) is released by the combustion of 12.50kilograms of propane in sufficient
Oxygen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning