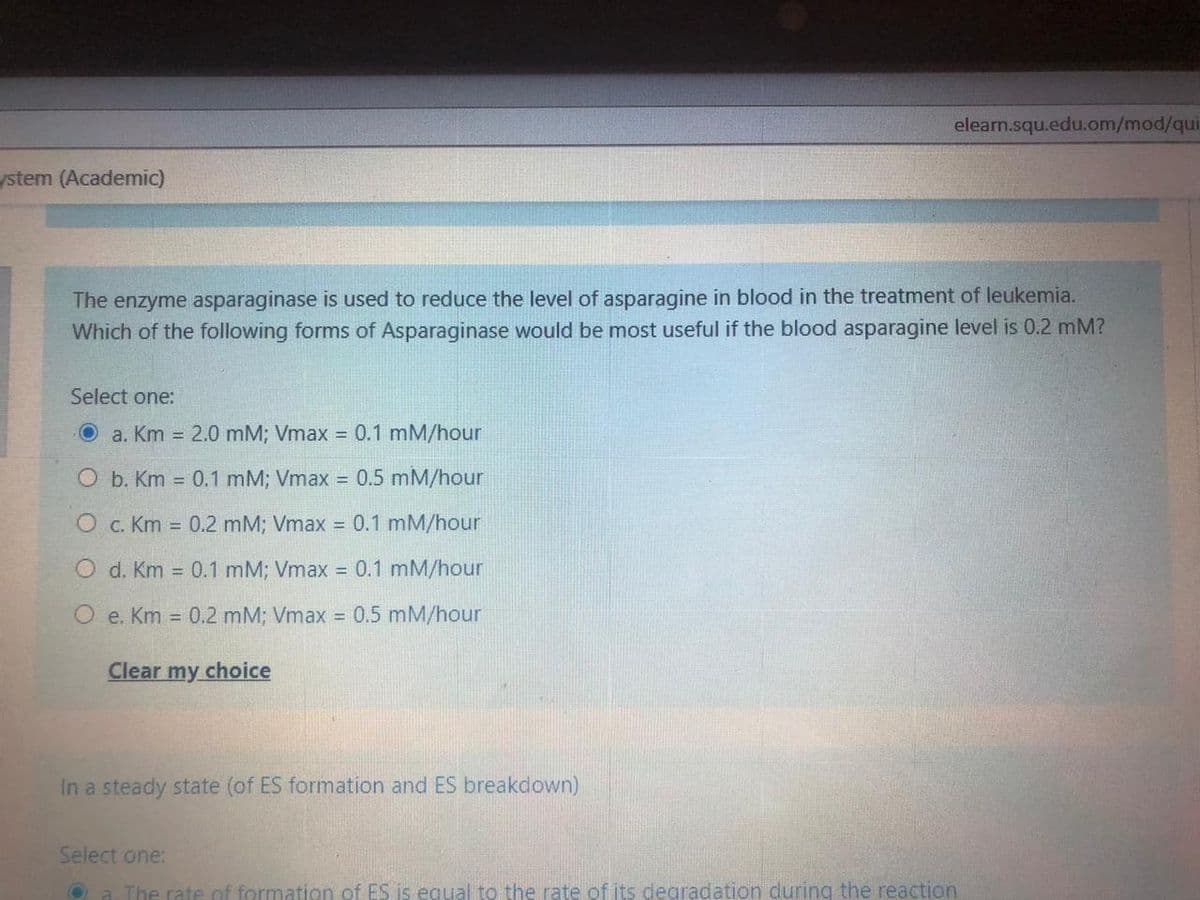
The enzyme asparaginase is used to reduce the level of asparagine in blood in the treatment of leukemia. Which of the following forms of Asparaginase would be most useful if the blood asparagine level is 0.2 mM? Select one: Oa. Km = 2.0 mM; Vmax = 0.1 mM/hour O b. Km = 0.1 mM; Vmax = 0.5 mM/hour O c. Km = 0.2 mM; Vmax = 0.1 mM/hour O d. Km = 0.1 mM; Vmax = 0.1 mM/hour O e. Km = 0.2 mM; Vmax = 0.5 mM/hour
The enzyme asparaginase is used to reduce the level of asparagine in blood in the treatment of leukemia. Which of the following forms of Asparaginase would be most useful if the blood asparagine level is 0.2 mM? Select one: Oa. Km = 2.0 mM; Vmax = 0.1 mM/hour O b. Km = 0.1 mM; Vmax = 0.5 mM/hour O c. Km = 0.2 mM; Vmax = 0.1 mM/hour O d. Km = 0.1 mM; Vmax = 0.1 mM/hour O e. Km = 0.2 mM; Vmax = 0.5 mM/hour
Biochemistry
9th Edition
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Chapter5: Protein Purification And Characterization Techniques
Section: Chapter Questions
Problem 39RE: RECALL The accompanying figure is from an electrophoresis experiment using SDSPAGE. The left lane...
Related questions
Question
Transcribed Image Text:elearn.squ.edu.om/mod/qui
ystem (Academic)
The enzyme asparaginase is used to reduce the level of asparagine in blood in the treatment of leukemia.
Which of the following forms of Asparaginase would be most useful if the blood asparagine level is 0.2 mM?
Select one:
O a. Km = 2.0 mM; Vmax = 0.1 mM/hour
O b. Km = 0.1 mM; Vmax = 0.5 mM/hour
%3D
О с. Кm
0.2 mM; Vmax = 0.1 mM/hour
O d. Km = 0.1 mM; Vmax = 0.1 mM/hour
O e. Km = 0.2 mM; Vmax = 0.5 mM/hour
Clear my choice
In a steady state (of ES formation and ES breakdown)
Select one:
a The rate of formation of ES is equal to the rate of its degradation during the reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning