The extinction coefficient for FD&C Blue Dye #1 is 138,500 cm-L/mol at 630 nm. A chemist collected the following data at 630 nm. The student also found that the absorbance of an FD&C Blue Dye #1 solution of unknown concentration was 0.561. Use your Vernier Graphical Analysis to generate a standard curve from this data set, and use it to determine the concentration of the unknown solution. (Report your final answer to 3 sig figs) Concentration of Blue Dye (M) Absorbance @ 630 nm 6.35x10-7 1.23x10-6 3.84x10-6 5.68x10-6 0.088 0.160 |0.541 0.787
The extinction coefficient for FD&C Blue Dye #1 is 138,500 cm-L/mol at 630 nm. A chemist collected the following data at 630 nm. The student also found that the absorbance of an FD&C Blue Dye #1 solution of unknown concentration was 0.561. Use your Vernier Graphical Analysis to generate a standard curve from this data set, and use it to determine the concentration of the unknown solution. (Report your final answer to 3 sig figs) Concentration of Blue Dye (M) Absorbance @ 630 nm 6.35x10-7 1.23x10-6 3.84x10-6 5.68x10-6 0.088 0.160 |0.541 0.787
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 77PS
Related questions
Question
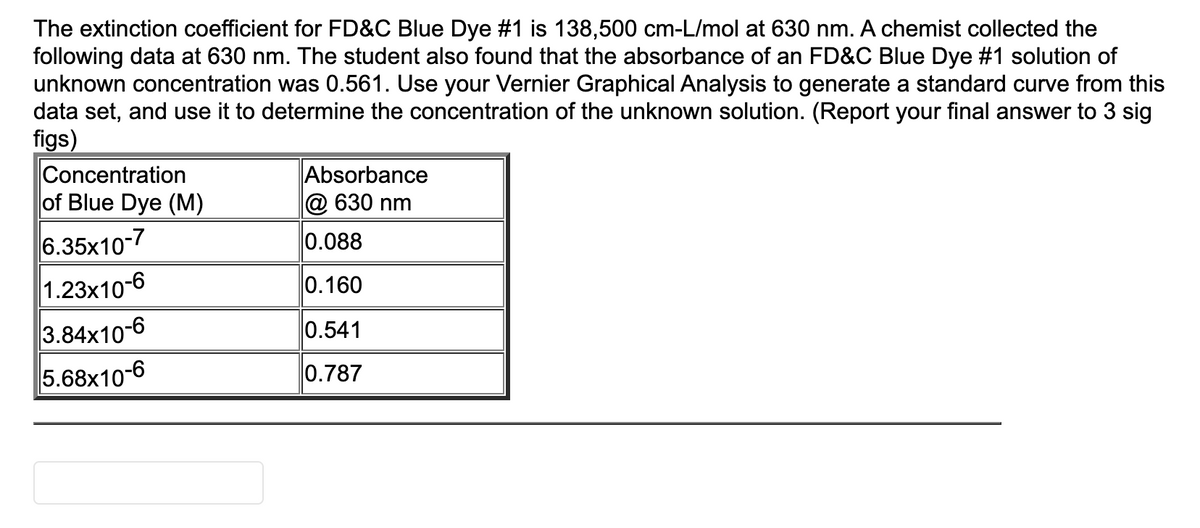
Transcribed Image Text:The extinction coefficient for FD&C Blue Dye #1 is 138,500 cm-L/mol at 630 nm. A chemist collected the
following data at 630 nm. The student also found that the absorbance of an FD&C Blue Dye #1 solution of
unknown concentration was 0.561. Use your Vernier Graphical Analysis to generate a standard curve from this
data set, and use it to determine the concentration of the unknown solution. (Report your final answer to 3 sig
figs)
Concentration
of Blue Dye (M)
Absorbance
@ 630 nm
6.35x10-7
0.088
1.23x10-6
0.160
3.84x10-6
0.541
5.68x10-6
0.787
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning