The figure above shows the absorption spectrum of the molecule HBr. Following the basic procedures of Section 9.6, find: (a) the energy of the “missing” transition; (b) the effective force constant k; (c) the rotational spacing 2B. Estimate the value of the rotational spacing expected for HBr and compare with the value deduced from the spectrum. Why are there only single lines and not double lines as in the case of HCl?
The figure above shows the absorption spectrum of the molecule HBr. Following the basic procedures of Section 9.6, find: (a) the energy of the “missing” transition; (b) the effective force constant k; (c) the rotational spacing 2B. Estimate the value of the rotational spacing expected for HBr and compare with the value deduced from the spectrum. Why are there only single lines and not double lines as in the case of HCl?
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter1: Introduction: The Nature Of Science And Physics
Section: Chapter Questions
Problem 15PE: (a) Suppose that a person has an average heart rate of 72.0 beats/min. How many beats does he or she...
Related questions
Question
The figure above shows the absorption spectrum of the molecule HBr. Following the basic procedures of Section 9.6, find:
(a) the energy of the “missing” transition;
(b) the effective force constant k;
(c) the rotational spacing 2B. Estimate the value of the rotational spacing expected for HBr and compare with the value deduced from the spectrum. Why are there only single lines and not double lines as in the case of HCl?
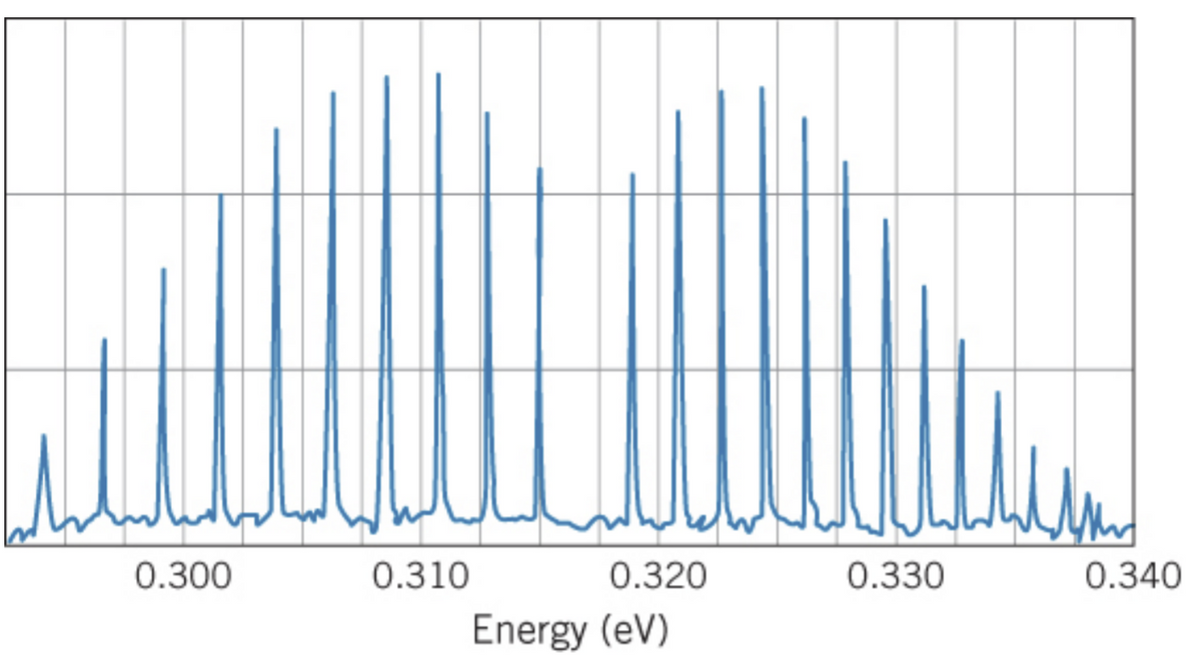
Transcribed Image Text:0.300
0.310
0.320
0.330
0.340
Energy (eV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College