The following are results from Part A of Lab 3. Identify the unknown metal cation Table 1: Observations for Qualitative Spot Tests Between Solutions Containing Single Metal Cation and Various Test Reagents from Part A Test Reagent K,Cr04 KSCN NH3 DMG HCI H2SO4 ppt, yellow, a ppt, white, ppt, white, a ppt, white, Pb2 NR NR some some lot lot ppt, CC, light CC, yellow CC, CC, dark ppt, brown, a Fe orange, colourless red lot brown some CC, light blue/violet ppt, pink, a lot Ni2+ NR NR NR NR ppt, white, a ppt, ppt, Ag* brown, a white, a NR NR NR lot lot lot ppt, yellow, a lot ppt, white, a ppt, white, Ba NR NR NR some lot K2Cr04 KSCN NH3 DMG HCI H2SO4 ppt, ppt, ppt, white, a lot Unknown brown, a NR NR white, a NR lot lot Select one: O Ag* O Pb2+ O Ni?+ O Fe3+ O Ba2+ O None of the Above Metal Cation Tested
The following are results from Part A of Lab 3. Identify the unknown metal cation Table 1: Observations for Qualitative Spot Tests Between Solutions Containing Single Metal Cation and Various Test Reagents from Part A Test Reagent K,Cr04 KSCN NH3 DMG HCI H2SO4 ppt, yellow, a ppt, white, ppt, white, a ppt, white, Pb2 NR NR some some lot lot ppt, CC, light CC, yellow CC, CC, dark ppt, brown, a Fe orange, colourless red lot brown some CC, light blue/violet ppt, pink, a lot Ni2+ NR NR NR NR ppt, white, a ppt, ppt, Ag* brown, a white, a NR NR NR lot lot lot ppt, yellow, a lot ppt, white, a ppt, white, Ba NR NR NR some lot K2Cr04 KSCN NH3 DMG HCI H2SO4 ppt, ppt, ppt, white, a lot Unknown brown, a NR NR white, a NR lot lot Select one: O Ag* O Pb2+ O Ni?+ O Fe3+ O Ba2+ O None of the Above Metal Cation Tested
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.18QAP
Related questions
Question
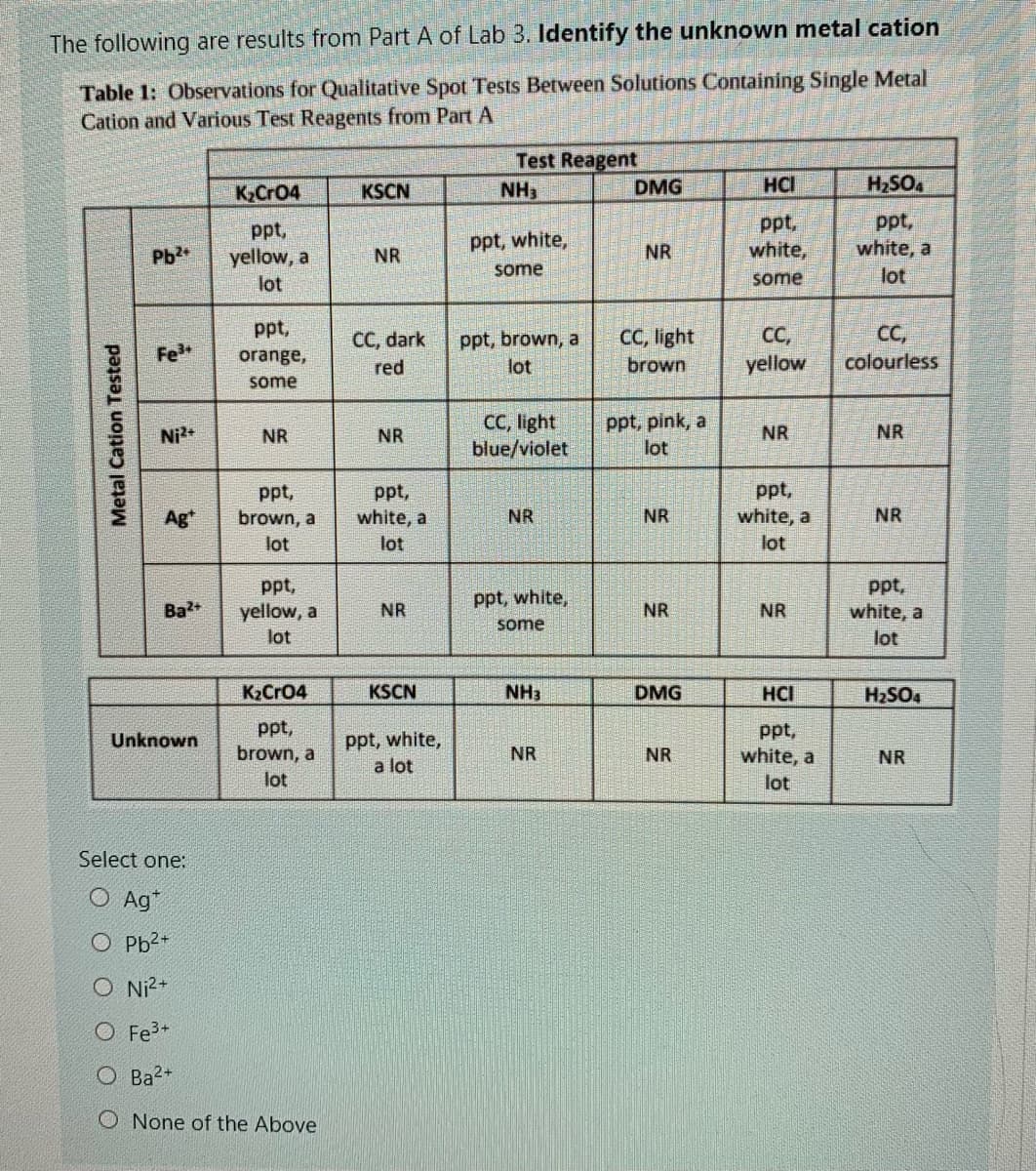
Transcribed Image Text:The following are results from Part A of Lab 3. Identify the unknown metal cation
Table 1: Observations for Qualitative Spot Tests Between Solutions Containing Single Metal
Cation and Various Test Reagents from Part A
Test Reagent
K,Cr04
KSCN
NH3
DMG
HCI
H2SO4
ppt,
yellow, a
ppt,
white,
ppt,
white, a
ppt, white,
Pb2
NR
NR
some
lot
lot
some
ppt,
CC, light
C,
yellow
CC,
CC, dark
ppt, brown, a
Fe
orange,
red
lot
brown
colourless
some
CC, light
blue/violet
ppt, pink, a
lot
Ni
NR
NR
NR
NR
ppt,
brown, a
ppt,
white, a
ppt,
Ag*
white, a
NR
NR
NR
lot
lot
lot
ppt,
white, a
ppt,
ppt, white,
Ba+
yellow, a
NR
NR
NR
some
lot
lot
K2Cr04
KSCN
NH3
DMG
HCI
H2SO4
ppt,
ppt, white,
a lot
ppt,
white, a
Unknown
brown, a
NR
NR
NR
lot
lot
Select one:
O Ag*
O Pb2+
O Ni?+
O Fe?+
O Ba2+
O None of the Above
Metal Cation Tested
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
