The following are two sets of data for the atomic mass of antimony the work of Willard and McAlpine Set 1 Set 2 121.771 121.784 121.787 121.758 121.803 121.765 121.781 121.794 a) Determine the 95% confidence interval for each data set. b) Determine whether the 121.803 value in the first data set is an outlier for that set at the 95% confidence level. c) Use the t test to determine whether the means of the two data sets differ at the 95% confidence level. d) Use the F test to determine whether the variances of the two data sets differ at the 95% confidence level.
The following are two sets of data for the atomic mass of antimony the work of Willard and McAlpine Set 1 Set 2 121.771 121.784 121.787 121.758 121.803 121.765 121.781 121.794 a) Determine the 95% confidence interval for each data set. b) Determine whether the 121.803 value in the first data set is an outlier for that set at the 95% confidence level. c) Use the t test to determine whether the means of the two data sets differ at the 95% confidence level. d) Use the F test to determine whether the variances of the two data sets differ at the 95% confidence level.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 43P
Related questions
Question
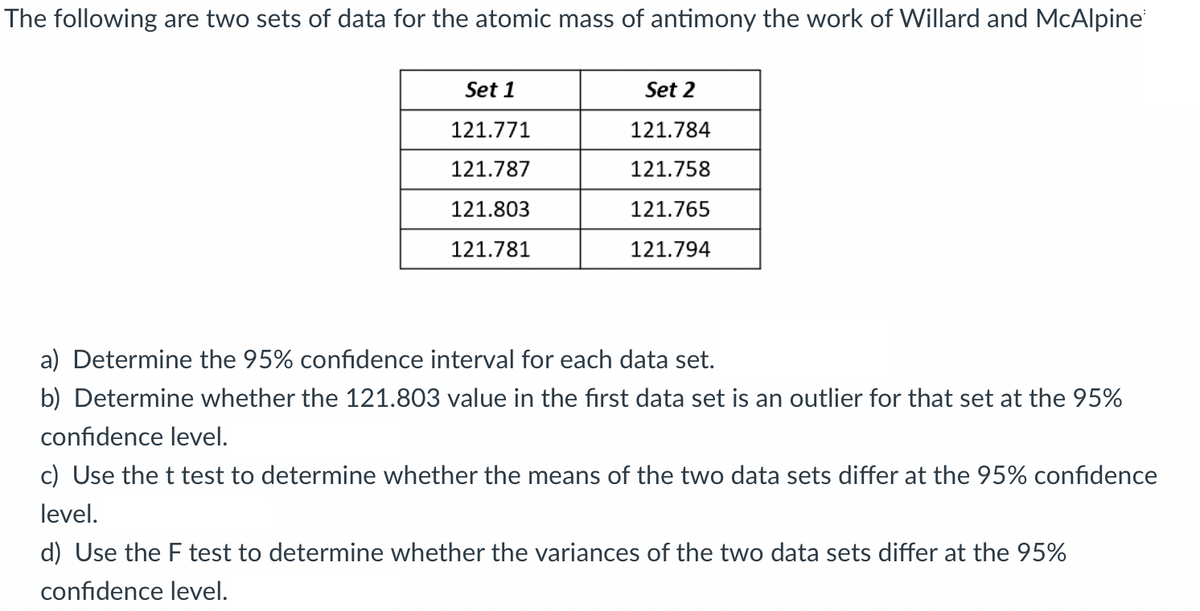
Transcribed Image Text:The following are two sets of data for the atomic mass of antimony the work of Willard and McAlpine
Set 1
Set 2
121.771
121.784
121.787
121.758
121.803
121.765
121.781
121.794
a) Determine the 95% confidence interval for each data set.
b) Determine whether the 121.803 value in the first data set is an outlier for that set at the 95%
confidence level.
c) Use the t test to determine whether the means of the two data sets differ at the 95% confidence
level.
d) Use the F test to determine whether the variances of the two data sets differ at the 95%
confidence level.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 8 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning