The following balanced equations represent reactions that occur in aqueous acid. Break them down into balanced oxidation and reduction half-equations. (a) 2 H;O*(aq) +H;O2(aq) + 2 Fe²* (aq) - 2 Fe** (aq) + 4 H2O(€) (b) H3O*(aq) + H2O(€) + 2 MnO (aq) + 5 SO2(aq) (aq) + 5 HSO, (aq) 2 Mn²+ (c) 5 CIO (aq) + 4 H;O* (aq) 4 CIO2(g) + CI¯(aq) + 6 H2O(e)
The following balanced equations represent reactions that occur in aqueous acid. Break them down into balanced oxidation and reduction half-equations. (a) 2 H;O*(aq) +H;O2(aq) + 2 Fe²* (aq) - 2 Fe** (aq) + 4 H2O(€) (b) H3O*(aq) + H2O(€) + 2 MnO (aq) + 5 SO2(aq) (aq) + 5 HSO, (aq) 2 Mn²+ (c) 5 CIO (aq) + 4 H;O* (aq) 4 CIO2(g) + CI¯(aq) + 6 H2O(e)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.108QE: At 298 K, the solubility product constant for solid Ba(IO3)2 is 1.5 109. Use the standard reduction...
Related questions
Question
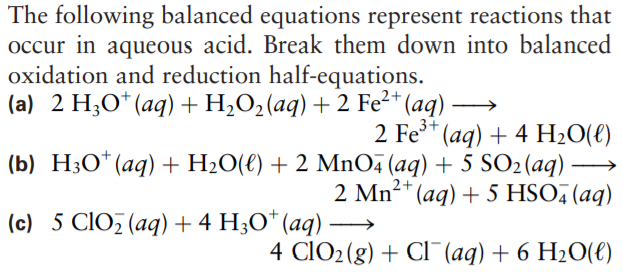
Transcribed Image Text:The following balanced equations represent reactions that
occur in aqueous acid. Break them down into balanced
oxidation and reduction half-equations.
(a) 2 H;O*(aq) +H;O2(aq) + 2 Fe²* (aq) -
2 Fe**
(aq) + 4 H2O(€)
(b) H3O*(aq) + H2O(€) + 2 MnO (aq) + 5 SO2(aq)
(aq) + 5 HSO, (aq)
2 Mn²+
(c) 5 CIO (aq) + 4 H;O* (aq)
4 CIO2(g) + CI¯(aq) + 6 H2O(e)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning