the following data were obtained from replicate 1.00g samples of cement dissolved in HCI and diluted to 100.0mL after neutralization Replicate I Replicate 2 Replicate 3 Blank 5.1 4.8 4.9 Sample A 28.6 28.2 28.9 Sample B 40.7 41.2 40.2 Sample C 73.1 72.1 spilled Calculate the percent Cr₂O3 in each sample. What are the absolute and relative standard deviations for the average of each determination?
the following data were obtained from replicate 1.00g samples of cement dissolved in HCI and diluted to 100.0mL after neutralization Replicate I Replicate 2 Replicate 3 Blank 5.1 4.8 4.9 Sample A 28.6 28.2 28.9 Sample B 40.7 41.2 40.2 Sample C 73.1 72.1 spilled Calculate the percent Cr₂O3 in each sample. What are the absolute and relative standard deviations for the average of each determination?
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.25QAP
Related questions
Question
Solve for me Any good expert there? Handwriting
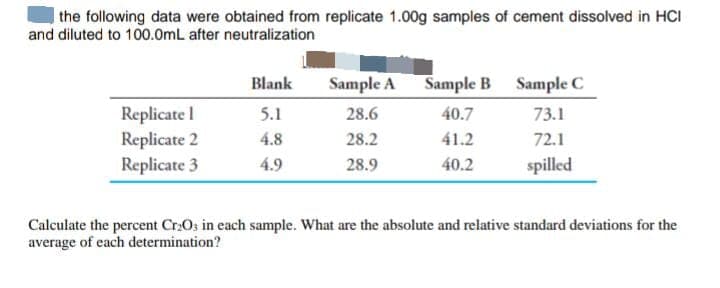
Transcribed Image Text:the following data were obtained from replicate 1.00g samples of cement dissolved in HCI
and diluted to 100.0mL after neutralization
Replicate I
Replicate 2
Replicate 3
Blank
5.1
4.8
4.9
Sample A
28.6
28.2
28.9
Sample B
40.7
41.2
40.2
Sample C
73.1
72.1
spilled
Calculate the percent Cr₂O3 in each sample. What are the absolute and relative standard deviations for the
average of each determination?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning