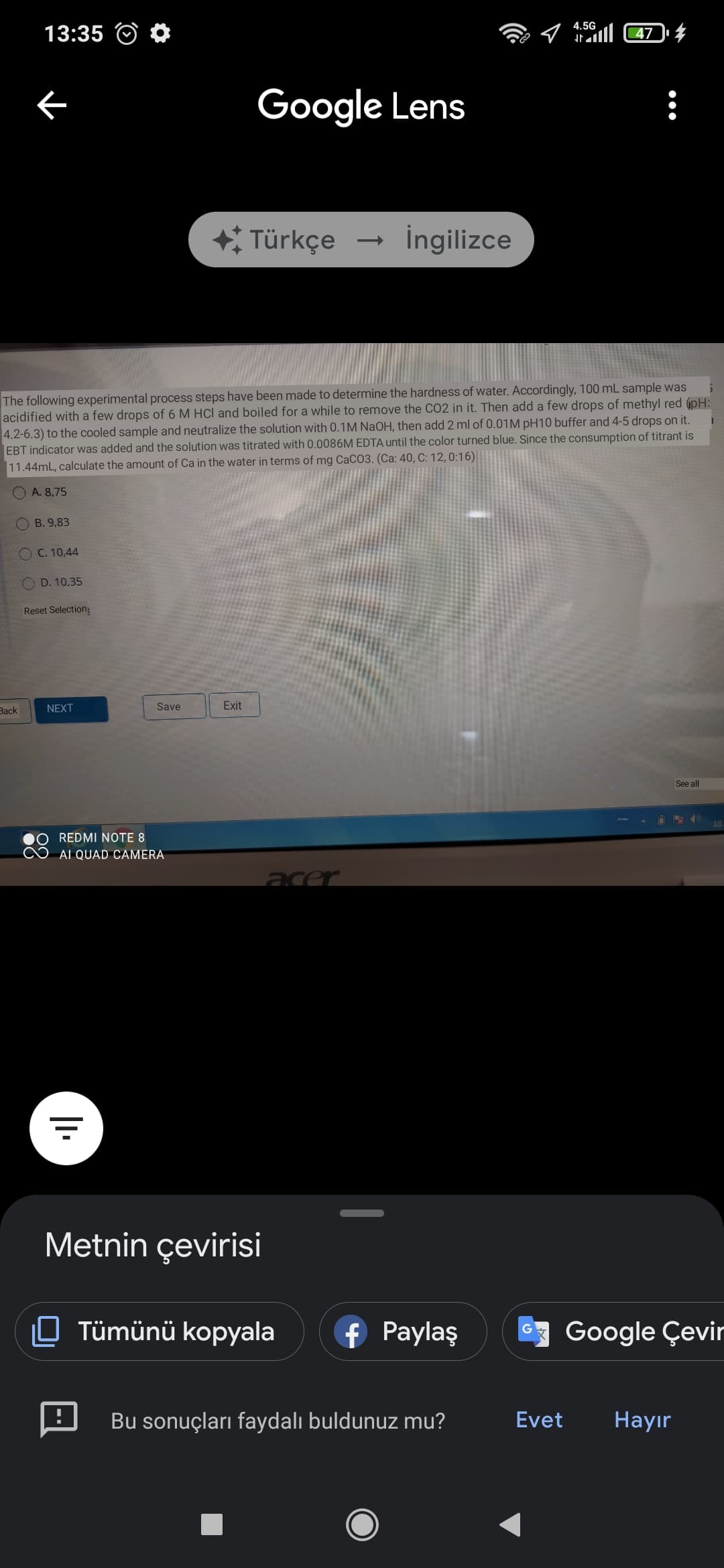
The following experimental process steps have been made to determine the hardness of water. Accordingly, 100 mL sample was acidified with a few drops of 6 M HCl and boiled for a while to remove the CO2 in it. Then add a few drops of methyl red 6pH: 4.2-6.3) to the cooled sample and neutralize the solution with 0.1M NaOH, then add 2 ml of 0.01M pH10 buffer and 4-5 drops on it. EBT indicator was added and the solution was titrated with 0.0086M EDTA until the color turned blue. Since the consumption of titrant is 11.44mL, calculate the amount of Ca in the water in terms of mg CaCO3. (Ca: 40, C: 12, 0:16) A. 8,75 O B. 9,83 C. 10,44 D. 10,35 Reset Selection
The following experimental process steps have been made to determine the hardness of water. Accordingly, 100 mL sample was acidified with a few drops of 6 M HCl and boiled for a while to remove the CO2 in it. Then add a few drops of methyl red 6pH: 4.2-6.3) to the cooled sample and neutralize the solution with 0.1M NaOH, then add 2 ml of 0.01M pH10 buffer and 4-5 drops on it. EBT indicator was added and the solution was titrated with 0.0086M EDTA until the color turned blue. Since the consumption of titrant is 11.44mL, calculate the amount of Ca in the water in terms of mg CaCO3. (Ca: 40, C: 12, 0:16) A. 8,75 O B. 9,83 C. 10,44 D. 10,35 Reset Selection
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
The following experimental process steps have been made to determine the hardness of water. Accordingly, 100 mL sample was acidified with a few drops of 6 M HCl and boiled for a while to remove the CO2 in it. Then add a few drops of methyl red (pH: 4.2-6.3) to the cooled sample and neutralize the solution with 0.1M NaOH, then add 2 ml of 0.01M pH10 buffer and 4-5 drops on it.
EBT indicator was added and the solution was titrated with 0.0086M EDTA until the color turned blue. Since the consumption of titrant is 11.44mL, calculate the amount of Ca in the water in terms of mg CaCO3. (Ca: 40, C: 12, 0:16)
Transcribed Image Text:4.5G
13:35
l 47
Google Lens
Türkçe
İngilizce
The following experimental process steps have been made to determine the hardness of water. Accordingly, 100 mL sample was
acidified with a few drops of 6 M HCl and boiled for a while to remove the CO2 in it. Then add a few drops of methyl red 6pH:
4.2-6.3) to the cooled sample and neutralize the solution with 0.1M NaOH, then add 2 ml of 0.01M pH10 buffer and 4-5 drops on it.
EBT indicator was added and the solution was titrated with 0.0086M EDTA until the color turned blue. Since the consumption of titrant is
11.44mL, calculate the amount of Ca in the water in terms of mg CaCO3. (Ca: 40, C: 12, 0:16)
O A. 8,75
B. 9,83
O C. 10,44
O D. 10,35
Reset Selection
Зack
NEXT
Save
Exit
See all
18
REDMI NOTE 8
AI QUAD CAMERA
acer
Metnin çevirisi
0 Tümünü kopyala
f Paylaş
Google Çevir
Bu sonuçları faydalı buldunuz mu?
Evet
Наyir
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

