The following is a proposed synthetic strategy that uses one starting alcohol in two separate single-step reactions (I and II) to make a pair of diene and dienophile which together undergo a Diels-Alder reaction to give a Diels-Alder product. The Diels-Alder product is subsequently converted to the final ether in a three-step synthesis (III, IV and V). Identify the reagent(s) from Table I required for each of those three sysnthese and place the number (A1, A2 ..., B1, B2..., or C1, C2 ...) that represents the reagent(s) in the given box right next to the corresponding step numbers (I to V) of the synthesis. DO NOT fill in any box with more than one number. (Note: This question is different from the previous synthesis question which allows two numbers for each box). You will be graded based on the number in each box. Do not fill in any single box with more than one number. Do not leave any box blank. (I) diene of OH (III, IV and V) Diesl-Alder product (II) dienophile
The following is a proposed synthetic strategy that uses one starting alcohol in two separate single-step reactions (I and II) to make a pair of diene and dienophile which together undergo a Diels-Alder reaction to give a Diels-Alder product. The Diels-Alder product is subsequently converted to the final ether in a three-step synthesis (III, IV and V). Identify the reagent(s) from Table I required for each of those three sysnthese and place the number (A1, A2 ..., B1, B2..., or C1, C2 ...) that represents the reagent(s) in the given box right next to the corresponding step numbers (I to V) of the synthesis. DO NOT fill in any box with more than one number. (Note: This question is different from the previous synthesis question which allows two numbers for each box). You will be graded based on the number in each box. Do not fill in any single box with more than one number. Do not leave any box blank. (I) diene of OH (III, IV and V) Diesl-Alder product (II) dienophile
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 46CTQ
Related questions
Question
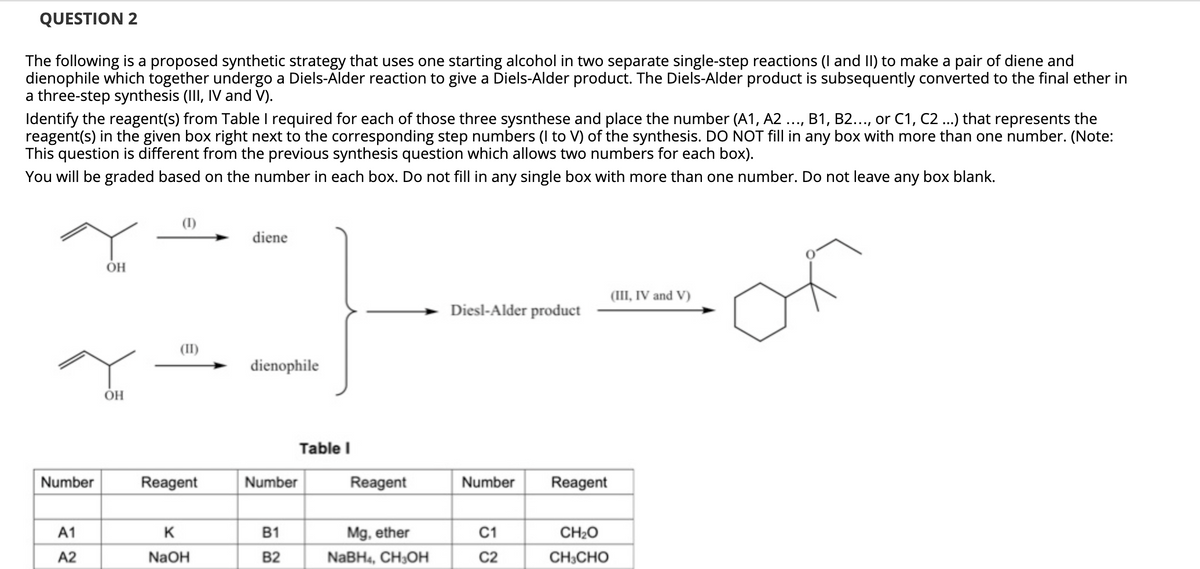
Transcribed Image Text:QUESTION 2
The following is a proposed synthetic strategy that uses one starting alcohol in two separate single-step reactions (I and II) to make a pair of diene and
dienophile which together undergo a Diels-Alder reaction to give a Diels-Alder product. The Diels-Alder product is subsequently converted to the final ether in
a three-step synthesis (III, IV and V).
Identify the reagent(s) from Table I required for each of those three sysnthese and place the number (A1, A2
reagent(s) in the given box right next to the corresponding step numbers (I to V) of the synthesis. DO NOT fill in any box with more than one number. (Note:
This question is different from the previous synthesis question which allows two numbers for each box).
You will be graded based on the number in each box. Do not fill in any single box with more than one number. Do not leave any box blank.
B1, B2..., or C1, C2 ...) that represents the
(I)
diene
OH
(III, IV and V)
Diesl-Alder product
(II)
dienophile
Table I
Number
Reagent
Number
Reagent
Number
Reagent
A1
K
B1
Mg, ether
C1
CH2O
A2
NaOH
B2
NaBH4, CH3OH
C2
CH3CHO
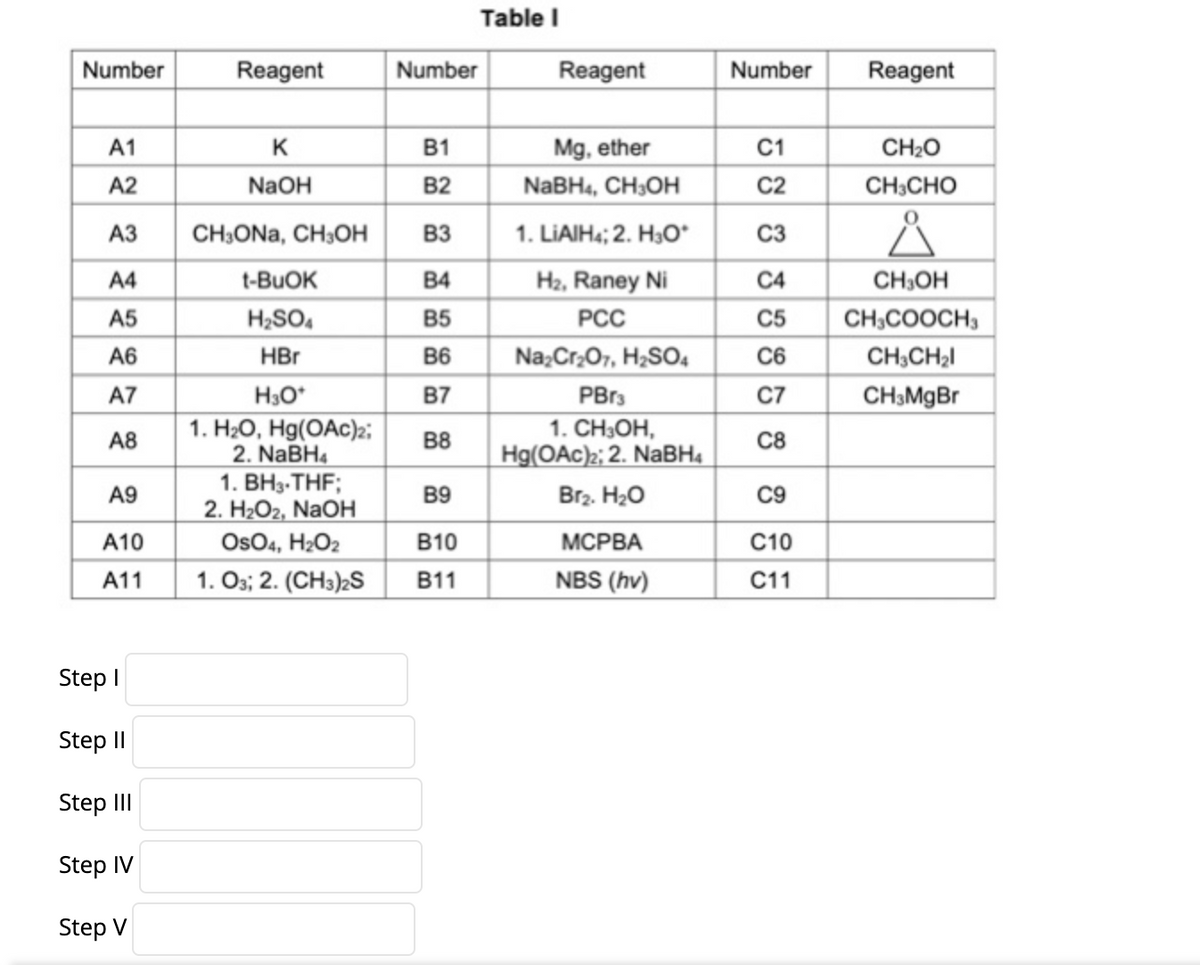
Transcribed Image Text:Table I
Number
Reagent
Number
Reagent
Number
Reagent
A1
K
B1
Mg, ether
C1
CH2O
A2
NaOH
B2
NABH4, CH;OH
C2
CH;CHO
АЗ
CH;ONa, CH3OH
B3
1. LIAIH4; 2. H3O*
C3
A4
t-BUOK
B4
H2, Raney Ni
C4
CH,OH
A5
H2SO,
B5
PCC
C5
CH,COOCH,
A6
HBr
B6
Na;Cr;07, H;SO4
C6
CH;CH2I
A7
H3O*
B7
PBr3
C7
CH3MgBr
1. H2O, Hg(OAc)2;
2. NaBH4
1. ВНз-THF;
2. H2O2, NAOH
Os04, H2O2
1. Os; 2. (CH3)2S
1. CH3OH,
A8
B8
C8
Hg(OAc);; 2. NaBH4
A9
B9
Br2. H20
C9
A10
В10
МСРВА
C10
A11
B11
NBS (hv)
C11
Step I
Step II
Step III
Step IV
Step V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning