The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n) v with a charge of If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n) v with a charge of If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 1E: Write the electronic configuration for the ions of the third period element that form monoatomic...
Related questions
Question
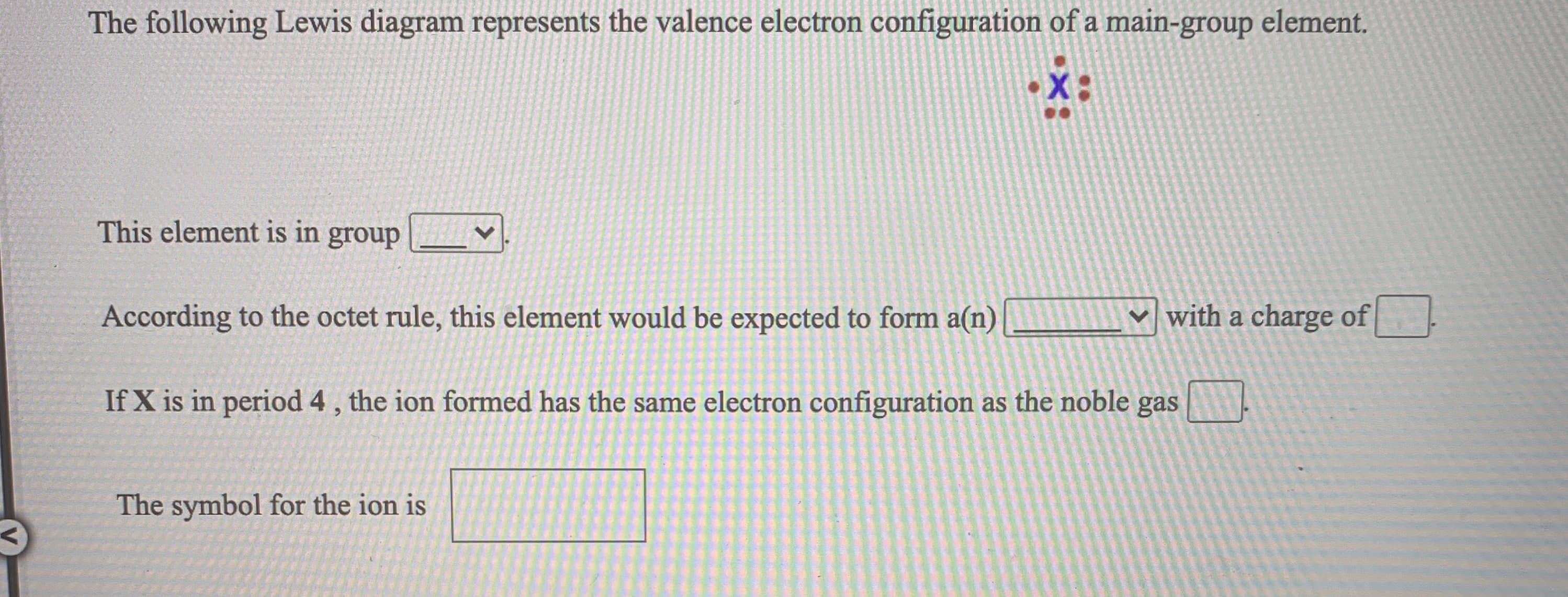
Transcribed Image Text:The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
According to the octet rule, this element would be expected to form a(n)
v with a charge of
If X is in period 4, the ion formed has the same electron configuration as the noble gas
The symbol for the ion is

Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning