The following model of phosphorus tribromide is wrong. Indicate WHY. Br -P-Br Br O There are too many electrons drawn in the molecule O There are not enough electrons drawn in the molecule O There are not the correct number of phosphorus atoms in the molecule O There are not the correct number of bromine atoms in the molecule
The following model of phosphorus tribromide is wrong. Indicate WHY. Br -P-Br Br O There are too many electrons drawn in the molecule O There are not enough electrons drawn in the molecule O There are not the correct number of phosphorus atoms in the molecule O There are not the correct number of bromine atoms in the molecule
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.14E: Write a symbol for each of the following ions: a.A selenium atom that has gained two electrons b.A...
Related questions
Question
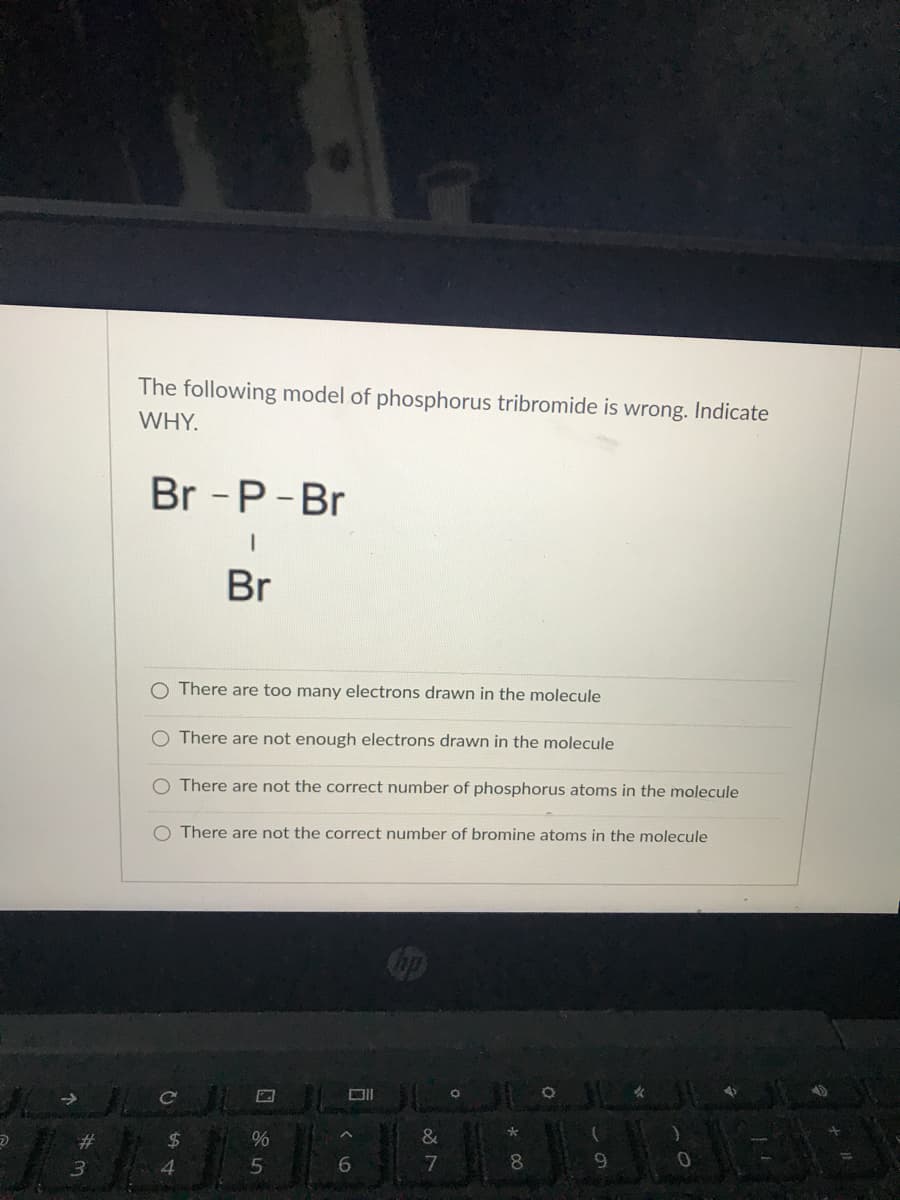
Transcribed Image Text:The following model of phosphorus tribromide is wrong. Indicate
WHY.
Br - P-Br
Br
O There are too many electrons drawn in the molecule
O There are not enough electrons drawn in the molecule
O There are not the correct number of phosphorus atoms in the molecule
O There are not the correct number of bromine atoms in the molecule
女
%23
24
4
7
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning