The following table shows the properties of five substances. Electrical conductivity when liquid дood good Melting point / °C 800 650 Effect of burning in Boiling point / °C 1470 Substance solid air No reaction Burns to form a А рor В 1110 good white solid 19 287 рoor poor Burns to form carbon dioxide and water Burns to form an acidic gas only No reaction 114 444 poor poor 1700 2200 рor рor Each substance can be used once, more than once or not at all to answer the following. Choose from A to E a substance which is: a) A metal b) A non-metallic element c) A simple molecular covalent compound d) An ionic compound e) A giant covalent substance
The following table shows the properties of five substances. Electrical conductivity when liquid дood good Melting point / °C 800 650 Effect of burning in Boiling point / °C 1470 Substance solid air No reaction Burns to form a А рor В 1110 good white solid 19 287 рoor poor Burns to form carbon dioxide and water Burns to form an acidic gas only No reaction 114 444 poor poor 1700 2200 рor рor Each substance can be used once, more than once or not at all to answer the following. Choose from A to E a substance which is: a) A metal b) A non-metallic element c) A simple molecular covalent compound d) An ionic compound e) A giant covalent substance
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.71PAE
Related questions
Question
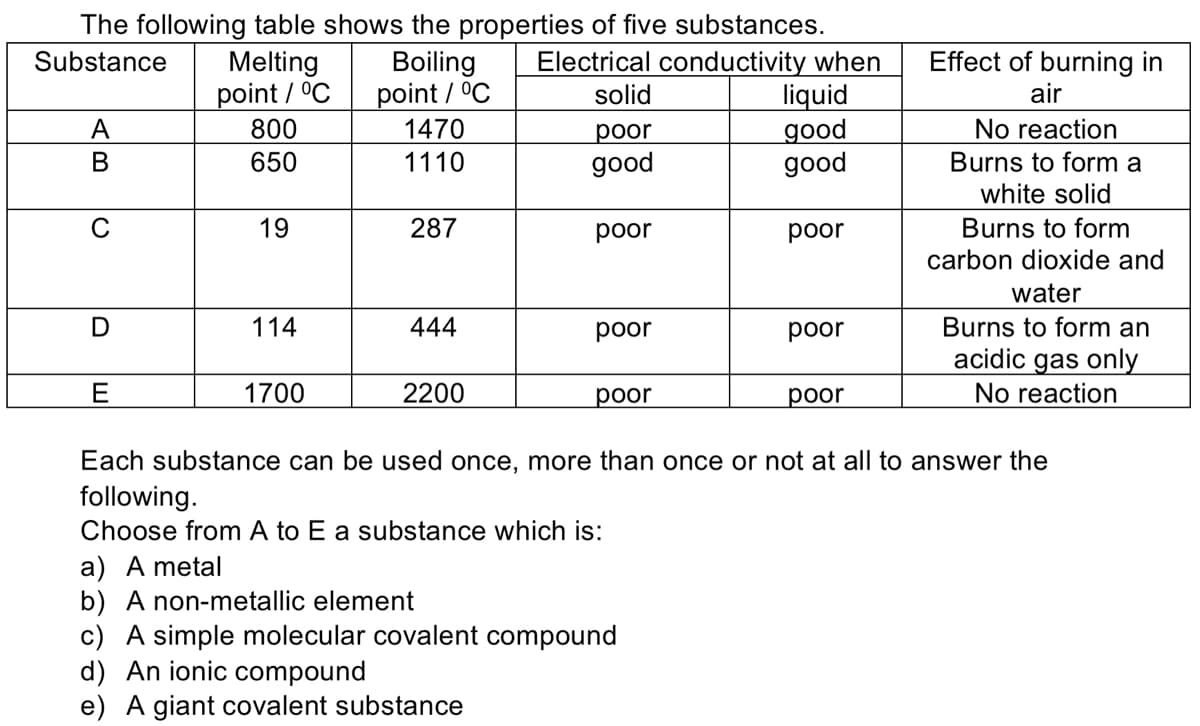
Transcribed Image Text:The following table shows the properties of five substances.
Electrical conductivity when
liquid
дod
good
Substance
Effect of burning in
Melting
point / °C
800
Boiling
point / °C
1470
solid
air
No reaction
Burns to form a
white solid
рor
В
650
1110
good
19
287
poor
poor
Burns to form
carbon dioxide and
water
114
444
рor
poor
Burns to form an
acidic gas only
E
1700
2200
рoor
рoor
No reaction
Each substance
be used once, more than once
not at all to answer the
following.
Choose from A to E a substance which is:
a) A metal
b) A non-metallic element
c) A simple molecular covalent compound
d) An ionic compound
e) A giant covalent substance
AB
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning