The formation of amenonia (NH occurs according to the following equation Na (9) + 3H2 (9) 2NHs (9) The following data was collected over a period of time Tune (s IN M 0) M INHL M 0.4 0. 1000 25 50 O 0500 0.035 025 01 0.205 013 75 0.0225 0012 0.1675 0.136 0.155 100 0.176 125 0.0008 0.1024 01984 150 O00065 0.10195 0.1987 Use the table to determine the rate of disappearance of hydrogen gas between 100 and 125 s o 744 x 10 M/s 22.38 x 10Ms O 134 x 10 M/s 1 420 x 10 M/s O 6.00 x 10 M/s
The formation of amenonia (NH occurs according to the following equation Na (9) + 3H2 (9) 2NHs (9) The following data was collected over a period of time Tune (s IN M 0) M INHL M 0.4 0. 1000 25 50 O 0500 0.035 025 01 0.205 013 75 0.0225 0012 0.1675 0.136 0.155 100 0.176 125 0.0008 0.1024 01984 150 O00065 0.10195 0.1987 Use the table to determine the rate of disappearance of hydrogen gas between 100 and 125 s o 744 x 10 M/s 22.38 x 10Ms O 134 x 10 M/s 1 420 x 10 M/s O 6.00 x 10 M/s
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
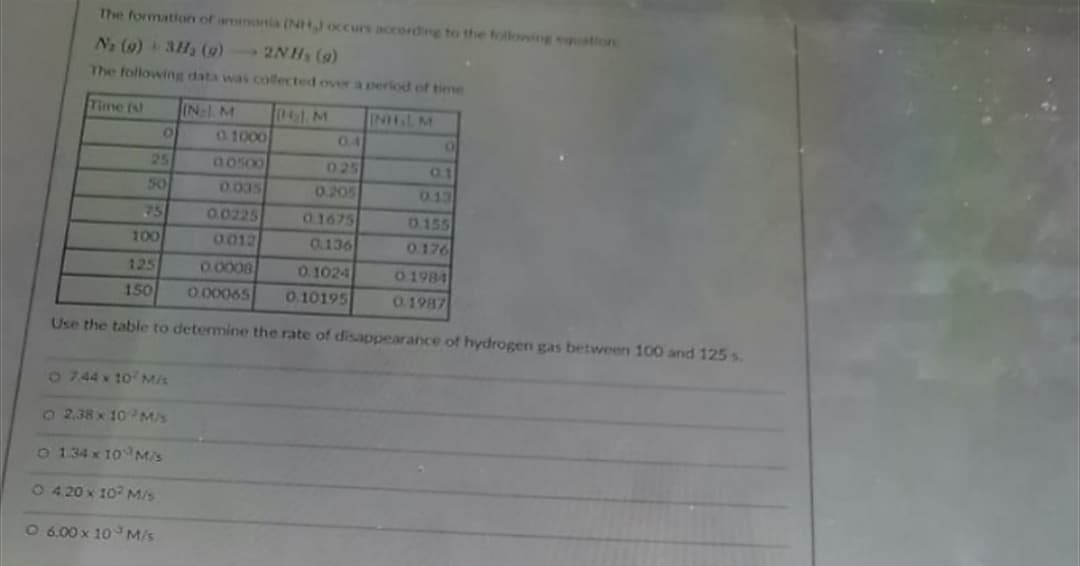
Transcribed Image Text:The formation of amnonia (NHJ occurs according to the tollowing equation
N2 (9) + 3H2 (9) 2NHs (9)
The following data was collected over a period of time
Time (s)
IN: M
(4) M
INHLM
0.4
0. 1000
25
O0500
025
0.1
50
0.035
0.205
0.13
25
0.0225
01675
0.155
100
0012
0.136
0.176
125
0.0008
0. 1024
01984
150
O 00065
0.10195
0.1987
Use the table to detemine the rate of disappearance of hydrogen gas between 100 and 125 s.
o744 x 10 M/s
22.38 x 10M/s
O 134 x 10 M/s
1420 x 10? M/s
O 6.00 x 10 M/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole