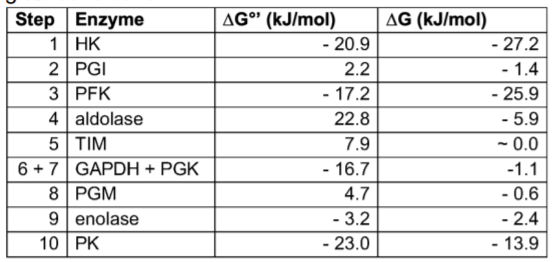
The free energy change of each step of glycolysis is given in the table below. ∆G°’ is the free energy under standard conditions (25°C, 1M each reactant, pH 7), while ∆G is the free energy change at presumed physiological conditions. Why must no step have a positive ∆G under physiological conditions?
Q: Phosphofructokinase (PFK) is the key regulatory enzyme of glycolysis. An Allosteric inhibitor of PFK…
A: Glycolysis is a major metabolic pathway which carry out the breakdown of glucose to form pyruvate…
Q: Consider the following reaction: CH,OPO CH,OPO CH-OH CH-OPO ATP ADP н но он phosphofructokinase-1…
A: In the third step of glycolysis, fructose-6-phosphate is converted to fructose-1,6-bisphosphate. An…
Q: Under standard conditions, NADH reoxidation by the electron-transport chain has a free-energy change…
A: ADP + Pi → ATP G= + 30.66 KJ /mol By the electron transport chain, NADH reoxidation has a free…
Q: From this enzymatic equation, a) identify each reaction as endergonic or exergonic, and b) briefly…
A: Spontaneous reaction favors the formation of products when two conditions, a decrease in enthalpy…
Q: What is the ΔG◦’ of ATP hydrolysis according to these data and is the overall reaction spontaneous?…
A: ∆G'° for any reaction is a fixed parameter as it is defined by standard condition. For spontaneous…
Q: A direct measurement of the standard free-energy change associated with the hydrolysis of ATP is…
A: ATP: Adenosine Tri-phosphate; NADPH: Nicotinamide adenine dinucleotide phosphate. These two are the…
Q: Consider the malate dehydrogenase reaction from the citric acid cycle. Given the listed…
A: The reaction catalyzed by malate dehydrogenase is given below. Malate + NAD+ ⇌ Oxaloacetate +…
Q: The complete combustion of palmitate and glucose yields 9781 kJ ∙ mol−1 and 2850 kJ ∙ mol−1 of free…
A: Cellular Respiration is the oxidative process through which energy is obtained from the food. The…
Q: ATP constitutes the most common cellular energy currency. Explain the factors that account for the…
A: The human body comprises a trillions number of cells. Every cell comprises mitochondria. The main…
Q: Why does this step in particular proceed spontaneously under typical cellular conditions?
A: Glycolysis is defined as a series of enzymatic reactions that convert one molecule of glucose, a…
Q: . If palmitic acid is subjected to complete combustion in a bomb calorimeter, one can calculate a…
A: The oxidation of the palmitic acid produces 8 acetyl Co-A + 7FADH2 + 7NADH in 7 cycles of the…
Q: In Bacillus subtilis, threonine is metabolized by the following sequence of reactions: (a)…
A:
Q: Upon digestion of starch, isomaltose (an isomer of maltose), one of its degradation products, is…
A: Aerobic metabolism is a set of three basic metabolic processes that occur in cells to generate…
Q: Glucose is phosphorylated to glucose-6-phosphate by hexokinase in the first step of the glycolytic…
A: Reactions that have a positive value of Delta G are not favorable in nature. Hence in order to…
Q: If a reaction has a ΔG°′ value of at least −30.5 kJ · mol−1, suffi -cient to drive the synthesis of…
A: The Standard Gibbs free energy change (∆G°) is the change in free energy when the substrate is…
Q: Nearly all organisms on Earth carry out some form of glycolysis. How does this fact support or not…
A: Prokaryotes may perform aerobic (oxygen-requiring) or anaerobic (non-oxygen-based) metabolism, and…
Q: The first reaction in Glycolysis is the phosphorylation of Glucose: Pi + Glucose - Glucose…
A: Glycolysis is a metabolic process that occurs in the cytoplasm irrespective of the presence or…
Q: What terms would best describe the above coupled reaction? (If the DGo for ATP hydrolysis into ADP +…
A: The Gibbs free energy change determines whether a biochemical reaction is favorable or not. It…
Q: Consider the following interconversion, which occurs in glycolysis :(a) What is ΔG′° for the…
A: Ans: Glycolysis: It is the process of breaking glucose in step wise manner to form pyruvate and CO2…
Q: Questión 73 ATP is used by cells for all of the following except
A: ATP ATP are adenosine triphosphate molecules which on hydrolysis releases water, inorganic…
Q: The regulation of 1-phosphofructokinase occurs primarily by allosteric effectors, including ATP and…
A: Glycolysis It is the 10 steps process of cellular respiration where one molecule of glucose is…
Q: The following reaction in glycolysis is catalyzed by the enzyme triose phosphate isomerase:…
A: Dihydroxyacetone phosphate and glyceraldehyde 3-phosphate are formed from fructose 1,6 bisphosphate…
Q: In a major metabolic pathway involving the monosaccharide glucose, one of the reactions involve the…
A: The given reaction represents the first reaction of the glycolysis pathway. In this reaction, the…
Q: One of the examples that we have used to illustrate the concept of equilibrium is the isomerization…
A: Mannose 6 phosphate is an enzyme which facilitates the interconversion of fructose 6 phosphate and…
Q: The standard free energy change for ATP hydrolysis is -7.3kcal/mol. The free energy change under…
A: Given; The standard free energy change for ATP hydrolysis is -7.3 kcal/mole. The free energy change…
Q: Organisms growing anaerobically cannot perform glycolysis for long without reducing the pyruvate…
A: During cellular respiration, respiratory substrates like glucose molecule can undergo a complete…
Q: Upon digestion of starch, isomaltose (an isomer of maltose), one of its degradation products, is…
A: Isomaltose is a glycosylglucose consisting of two D-glucopyranose units connected by an…
Q: The intermediates of glycolysis with a free energy of hydrolysis sufficient to couple conversion of…
A: Glycolysis is an oxidative process, in which glucose is partially oxidized into pyruvate in a series…
Q: Given the following information, calculate the physiological ΔG of the isocitrate dehydrogenase…
A: Gibbs free energy or Free energy is a potential of thermodynamics that helps in calculating…
Q: enzyme malate dehydrogenase catalyzes the conversion of malate to oxaloacetate in the final step…
A: In a reaction if a molecule is oxidized then other molecule must be reduced. This is called as…
Q: In the third step of glycolysis, the given reactions are coupled. reaction 1: fructose-6-phosphate…
A: The free energy change of a reaction or the Gibbs free energy or delta G can tell us whether or not…
Q: Consider the following mechanism: What kind of reaction is occurring during this step of glycolysis?…
A: "Since you have asked multiple subparts questions, we will solve the first five question for you. If…
Q: Glycolysis and Gluconeogenesis are effectively two sides of the same coin”. With the aid of a…
A: The first step in the cellular respiration is the glycolysis which involves the splitting of a…
Q: If palmitic acid is subjected to complete combustion in a bomb calorimeter, one can calculate a…
A: The oxidation of the palmitic acid produces 8 acetyl Co-A + 7FADH2 + 7NADH in 7 cycles of the…
Q: What is the net equation for the first 5 reactions of glycolysis?
A: Glycolysis is breakdown of glucose into pyruvate(aerobic oxidation) or lactate(anaerobic oxidation).…
Q: In glycolysis, the KM value of hexoquinase is 0.04 mM. At physiological conditions, the cellular…
A: Enzymes bind reactants (substrates) to their active sites and convert them into products. They…
Q: Otto Warburg made an interesting observation in the 1930s about cancer cells using the fermentation…
A: The reactive oxygen species (ROS) like superoxide ion is produced in the mitochondria under two…
Q: When glucose is used as the starting material for glycolysis there is a net gain of +2 ATP per…
A: Energy is needed for all functions and processes of the cell. Metabolic pathways play an important…
Q: n the living cell, free energy made from one reaction can be used to drive another in an…
A: Glycolysis is also known as the Embden Meyerhof pathway and it is highly conserved from…
Q: The enzyme malate dehydrogenase catalyzes the conversion of malate to oxaloacetate in the final step…
A: The malate dehydrogenase enzyme catalyzes the conversion of malate to oxaloacetate in the TCA cycle.…
Q: Consider the following phosphoryl group transfer reaction which is the first step in glucose…
A: The cell's initial mechanism for breaking down glucose for energy is called "glycolysis." There are…
Q: During cellular respiration, for each single glucose molecule 10 NADH molecules and 2 FADH2…
A: During glycolysis and citric acid cycle, much of the free energy is retained in the form of reduced…
Q: What percentage of ATP energy is produced when 9.0 moles of glucose are oxidized into acetyl CoA?…
A: Glycolysis generates net ATP = 2 ATP per mole of Glucose molecule Then end product of glycolysis -…
Q: ATP Synthase is known to catalyze the synthesis of ATP with a ΔG°’ close to zero, and a Keq' close…
A: The F1/F0 ATP synthase catalyzes the conversion of ADP to ATP coupled with the movement of protons…
Q: For a lot of enzymes that work on fatty acids, the rate determining step is the release of the…
A: Firstly, fatty acid synthesis is synthesis of fatty acids from acetyl-CoA and also utilizing NADPH.…
The free energy change of each step of glycolysis is given in the table below. ∆G°’ is the free energy under standard conditions (25°C, 1M each reactant, pH 7), while ∆G is the free energy change at presumed physiological conditions. Why must no step have a positive ∆G under physiological conditions?
Step by step
Solved in 2 steps with 2 images

- A new drug, Proinebrium, that reduces Kcat (Ki = 2.0 uM) has been developed to treat ethylene glycol poisoning. (1) What concentration of Proinebrium is required to achieve 50% inhibition of ethylene glycol metabolism by alcohol dehydrogenase when the concentraion of ethlyene glycol in the blood is 50 uM?Calculcate Kcat for PNP substrate for both enzyme concentrations. enzyme volume: 20 ul Bovine Intensince Alkaline phosphatase molecular weight: 140,000 Bovine intenstine Alkaline phosphatase activity: 300 units/ml and 14 units/mg extinction coefficient PNP: 18.5 abs (mM-1 cm-1) Vmax: 0.332 moles/sec a) enzyme 1 concentration: undiluted b) enzyme 2 concentration: 1:1 dilutionFor the following aspartase reaction in the presence of the inhibitor hydroxymethylaspartate, determine Km and whether the inhibition is competitive or noncompetitive. You have to plot thegraph on the graph paper and also by using excel.[S] V, No Inhibitor V, Inhibitor Present(molarity) (arbitrary units) (same arbitrary units) 1 x 10-4 0.026 0.0105 x 10-4 0.092 0.0401.5 x 10-3 0.136 0.0862.5 x 10-3 0.150 0.1205 x 10-3 0.165 0.142
- 15. G + C Content: Describe how the G+C content of an organism can be measured. In your response, be sure to address the following:a. What is the significance of determining GC vs. AT content?Calculate KI' of the inhibitor from the information given. All information may not be needed to calculate. K'm = (29Ki+1.45x10^-10)/Ki Vmax = 11.7 µMs-1 Kcat = 130 s^-1 Vo = 3.0 μMs-1 S = 10 μM Et = 0.09 µM Inhibitor Concentration = 5x10^-12Greg Tobin mixes together three types of vitamintablets. Each Super Vim tablet contains, among other things,15 mg of niacin and 12 I.U. of vitamin E. The figures for aMultitab tablet are 20 mg and 15 I.U., and for a Mighty Mix are25 mg and 35 I.U. How many of each tablet are there if the totalnumber of tablets, total amount of niacin, and total amount ofvitamin E are as follows?a. 225 tablets, 4750 mg of niacin, and 5225 I.U. of vitamin Eb. 185 tablets, 3625 mg of niacin, and 3750 I.U. of vitamin Ec. 230 tablets, 4450 mg of niacin, and 4210 I.U. of vitamin E
- A particular enzyme has a ΔΔG‡ of -22.1 kJ mol-1 at 37.0 °C. Calculate the rate enhancement of this enzyme. (R = 8.3145 J mol-1 K-1)In 2-page worth of words (around 500), discuss CYCLAMATE's regulation, allowable levels, and what group of people are at high risk for the side effects of CYCLAMATE in the body.Question 1. a) Explain how 5 specific fatty acids ultimately generate specific classes of prostaglandins and leukotrienes that are involved in blood pressure, platelet aggregation and inflammation.b) Indicate and explain the specific effects of each of these classes of prostaglandins and leukotrienes on blood pressure, platelet aggregation and inflammation.c) Identify which foods, functional foods and nutraceuticals provide one or more of these 5 fatty acids.
- Acetocholinesterase is an enzyme possessing a single active site that metabolizesacetylcholine with a turn over number of 1.4 x 10^4s-1. How many grams of acetylcholine(molecular formula C7NO2H16+) will 2.16 x 10^-6 g acetocholinesterase metabolize in 60minutes? (The enzyme’s molecular mass is 4.2 x 10^4 g/mol).The text describes a form of gout that results from HGPRT deficiency. Propose one or two additional enzyme abnormalities that might similarly lead to hyperuricemia.11.96 Is there a crude association between either measure of adiposity (BMI, WHR), considered separately, and serum estradiol?

