The graph shows the boiling points of various substances. Does SiH4 or CH4 have stronger intermolecular forces? Explain how you know, based on the graph and what you have learned about intermolecular forces. The trend in the data between the first and second molecules in Group 14 is different than in the other groups. How can you explain this difference in terms of intermolecular forces? Write a claim about the relative strengths of the different types of intermolecular forces. Support your claim with evidence from the graph above and what you have learned about intermolecular forces.
The graph shows the boiling points of various substances. Does SiH4 or CH4 have stronger intermolecular forces? Explain how you know, based on the graph and what you have learned about intermolecular forces. The trend in the data between the first and second molecules in Group 14 is different than in the other groups. How can you explain this difference in terms of intermolecular forces? Write a claim about the relative strengths of the different types of intermolecular forces. Support your claim with evidence from the graph above and what you have learned about intermolecular forces.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter10: The Mole
Section10.5: Formulas Of Hydrates
Problem 78SSC
Related questions
Question
100%
The graph shows the boiling points of various substances. Does SiH4 or CH4 have stronger intermolecular forces? Explain how you know, based on the graph and what you have learned about intermolecular forces.
The trend in the data between the first and second molecules in Group 14 is different than in the other groups. How can you explain this difference in terms of intermolecular forces?
Write a claim about the relative strengths of the different types of intermolecular forces. Support your claim with evidence from the graph above and what you have learned about intermolecular forces.
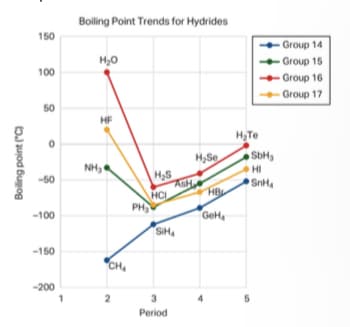
Transcribed Image Text:Boling Point Trends for Hydrides
150
Group 14
H,0
Group 15
100
Group 16
Group 17
50
HF
HyTe
H,Se
SbH
NH,
HI
-60
ASH
SnH
HC
HB
PH
-100
GeH
SH
-150
CH
-200
3
Period
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co