E. A chemical change involves a change in the composition of matter-the formation of a new chemical substance (product) with physical and chemical properties different from those of the reactants. Describe the evidence used to decide if any of the processes in Part A were chemical changes. Did you observe any qualitative differences in the amount of heat generated in the reac- tions that were characterized as endothermic in Part A? Consider Reaction A: Was energy released or absorbed by the reactants in this system? When you touched the reaction container (the plastic bag) was energy being released or absorbed by your hand? Write a balanced equation for each of the processes in Part A. Remember to include heat on the reactant or product side, as appropriate.
E. A chemical change involves a change in the composition of matter-the formation of a new chemical substance (product) with physical and chemical properties different from those of the reactants. Describe the evidence used to decide if any of the processes in Part A were chemical changes. Did you observe any qualitative differences in the amount of heat generated in the reac- tions that were characterized as endothermic in Part A? Consider Reaction A: Was energy released or absorbed by the reactants in this system? When you touched the reaction container (the plastic bag) was energy being released or absorbed by your hand? Write a balanced equation for each of the processes in Part A. Remember to include heat on the reactant or product side, as appropriate.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 74QAP: In 2010, 3.30109 gallons of gasoline were consumed in the United States. The following assumptions...
Related questions
Question
To ensure that you are not confused, I attached what the experiment was about. I highly recommend you read through it before going to the questions.
file:///Users/michaelzheng/Downloads/ExploringEnergy%20(2).pdf
Only answer Post lab questions 2-5
For number five, put the answers in the format stated in the background.
Thank you.
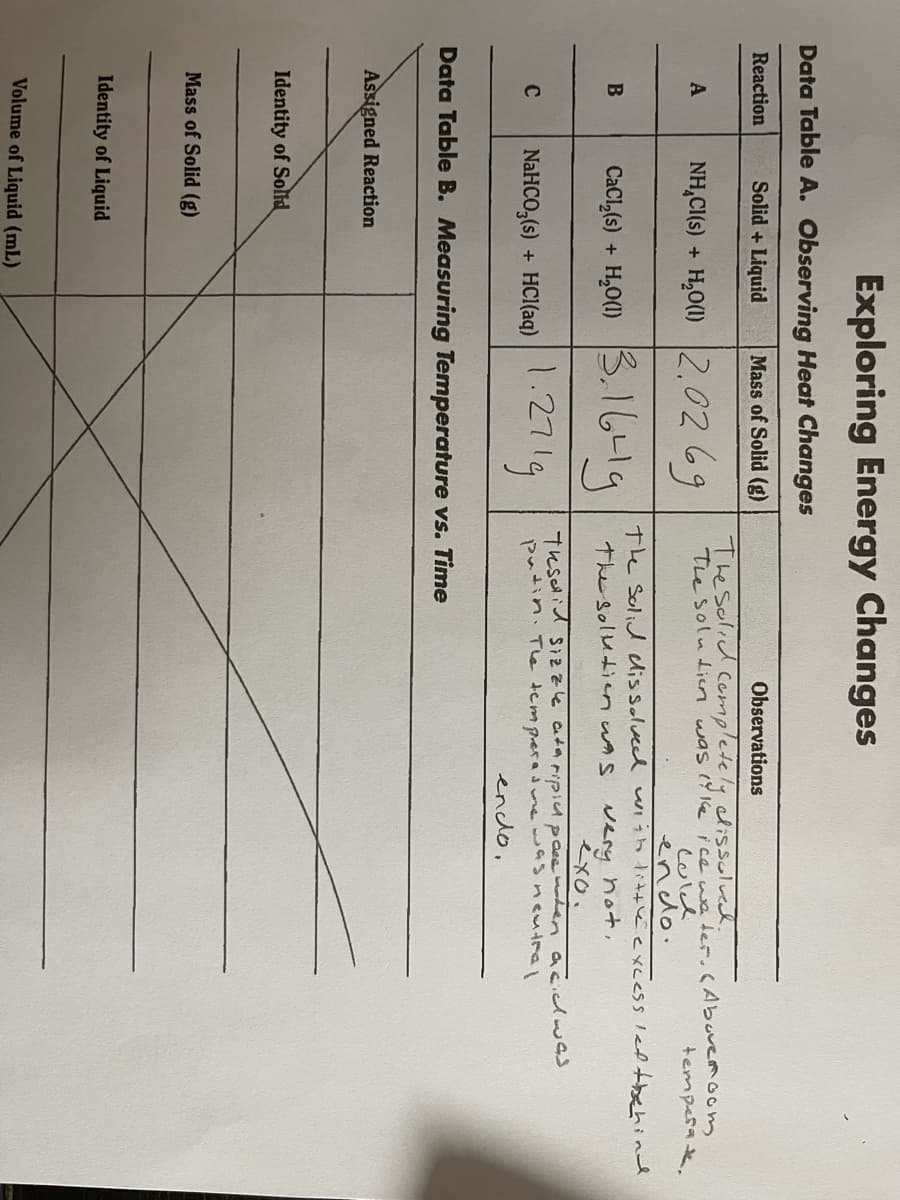
Transcribed Image Text:Exploring Energy Changes
Data Table A. Observing Heat Changes
Reaction
Solid + Liquid
Mass of Solid (g)
Observations
Thesolid Cempletely dissolved.
The soluien was ( 1e ice we ter.CAbuvemoom
Culd
endo.
The Solid lissolveed uiih little excess led tbehind
NH,CI(s) + H,O(1) 2.0269
A
temperak.
CacıĻ6) + 6-l9
H,O(1) 3.1
3.1641g
very hot,
exo.
The solutien wAs
NaHCO,(6) + HCl(aq) 271g
Tesid Sizzle ataripicA pase er acidwas
putin. Tle tempera mewasncutral
enclo,
C
NaHCO,(s) + HCI(aq)
Data Table B. Measuring Temperature vs. Time
Assigned Reaction
Identity of Solid
Mass of Solid (g)
Identity of Liquid
Volume of Liquid (mL)

Transcribed Image Text:Exploring Energy Changes-Page 6
Post-Lab Questions
Attach the printout of the data table and graph for Part B to your lab report.
1. Complete the following Results Table to indicate whether each reaction in Part A repre-
sents a physical or chemical change and whether it is exothermic or endothermic.
Reaction
Physical or Chemical Change?
Exothermic or Endothermic?
Physical Charge
Physical ihangE
Chemical.change
Endothermi
Exothermic
A
C
Endathermicc
2. A chemical change involves a change in the composition of matter-the formation of a
new chemical substance (product) with physical and chemical properties different from
those of the reactants. Describe the evidence used to decide if any of the processes in Part
A were chemical changes.
3. Did you observe any qualitative differences in the amount of heat generated in the reac-
tions that were characterized as endothermic in Part A?
4. Consider Reaction A: Was energy released or absorbed by the reactants in this system?
When you touched the reaction container (the plastic bag) was energy being released or
absorbed by your hand?
5. Write a balanced equation for each of the processes in Part A. Remember to include heat
on the reactant or product side, as appropriate.
6. In Part B, was the temperature that was measured a part of the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning