Use the Rakerences to aecens important valuas if naeded for dis quastion. The temperature dependence of the reaction rate constant is given by the equation: E, Ink= In A- RT Where k rate constant T= temperature A= frequency factor E, – Energy of aetivation In order to sove for the Energy of activation, E., you must: Sle One Add the same enpression to each side of the equation to lesve the term that inchludes the variable by itself on the right-hand side of the expression: (Be sure that the answer feld changes from light yellow to dark pellow before neleazing your answer.) E. + Ink- + In A- Drag and drop your sdlection from the following list to complete the answer In A - In A
Use the Rakerences to aecens important valuas if naeded for dis quastion. The temperature dependence of the reaction rate constant is given by the equation: E, Ink= In A- RT Where k rate constant T= temperature A= frequency factor E, – Energy of aetivation In order to sove for the Energy of activation, E., you must: Sle One Add the same enpression to each side of the equation to lesve the term that inchludes the variable by itself on the right-hand side of the expression: (Be sure that the answer feld changes from light yellow to dark pellow before neleazing your answer.) E. + Ink- + In A- Drag and drop your sdlection from the following list to complete the answer In A - In A
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 6P
Related questions
Question
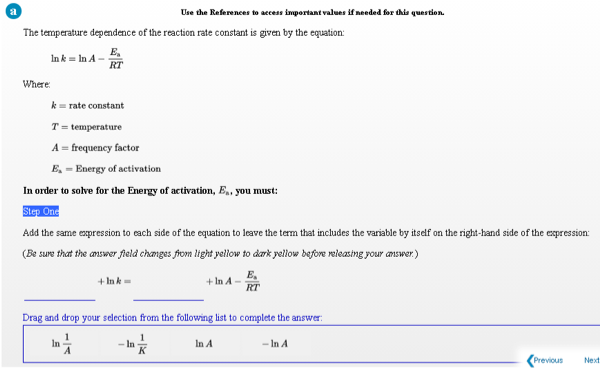
Transcribed Image Text:Use the References to access inmportant values if needed for this question.
The temperature dependence of the reaction rate constant is given by the equation:
E,
Ink = In A -
RT
Where:
k= rate constant
T = temperature
A = frequency factor
E, = Energy of activation
In order to solve for the Energy of activation, E.. you must:
Step One
Add the same expression to each side of the equation to leave the term that includes the variable by itself on the right-hand side of the expression:
(Be sure that the answer field changes fromn light yellow to dark yellow before releasing your answer.)
E,
+ Ink-
+ In A -
RT
Drag and drop your selection from the following list to complete the answer:
In
- In
In A
- In A
Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,