The ideal gas law describes the relationship among the pressure P, volume V, number of moles n., and absolute temperature T of an ideal gas. Here is the relationship expressed mathematically: PV = nRT where R is a proportionality constant. The units of R are determined by the units of pressure and volume used in the equation. When bar is used for pressure and I for volume, the appropriate R value is 0.08314 L bar mol-¹ K-¹ Part A How many air molecules are in a 4.05 x 3.66 x 3.05 m³ room? Assume atmospheric pressure of 1.00 bar, a room temperature of 20.0°C, and ideal behavior. Express your answer using three significant figures. ▸ View Available Hint(s) IVE] ΑΣΦ → O ? molecules
The ideal gas law describes the relationship among the pressure P, volume V, number of moles n., and absolute temperature T of an ideal gas. Here is the relationship expressed mathematically: PV = nRT where R is a proportionality constant. The units of R are determined by the units of pressure and volume used in the equation. When bar is used for pressure and I for volume, the appropriate R value is 0.08314 L bar mol-¹ K-¹ Part A How many air molecules are in a 4.05 x 3.66 x 3.05 m³ room? Assume atmospheric pressure of 1.00 bar, a room temperature of 20.0°C, and ideal behavior. Express your answer using three significant figures. ▸ View Available Hint(s) IVE] ΑΣΦ → O ? molecules
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
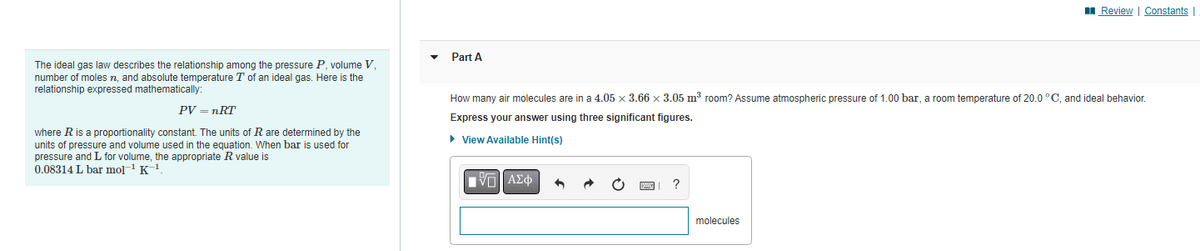
Transcribed Image Text:The ideal gas law describes the relationship among the pressure P, volume V,
number of moles n, and absolute temperature T of an ideal gas. Here is the
relationship expressed mathematically:
PV = nRT
where R is a proportionality constant. The units of R are determined by the
units of pressure and volume used in the equation. When bar used for
pressure and L for volume, the appropriate R value is
0.08314 L bar mol-¹ K-¹.
Part A
How many air molecules are in a 4.05 x 3.66 x 3.05 m³ room? Assume atmospheric pressure of 1.00 bar, a room temperature of 20.0 °C, and ideal behavior.
Express your answer using three significant figures.
► View Available Hint(s)
VE ΑΣΦ +
Review | Constants |
molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning