Question: The relationship between temperature and volume of a gas is linear and given by Charl's law V (ml) = Constant × T (°C) T(°C) -23 -13 -3 7 17 27 37 47 57 67 77 V (ml) 8.41525 8.75186 9.08847 9.42508 9.76169 10.09830 10.43491 10.77152 11.10813 11.44474 11.78135 Calculate the temperature (°C) at which the volume of the gas become close to zero.
Question: The relationship between temperature and volume of a gas is linear and given by Charl's law V (ml) = Constant × T (°C) T(°C) -23 -13 -3 7 17 27 37 47 57 67 77 V (ml) 8.41525 8.75186 9.08847 9.42508 9.76169 10.09830 10.43491 10.77152 11.10813 11.44474 11.78135 Calculate the temperature (°C) at which the volume of the gas become close to zero.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.18E: Scottish physicist W. J. M. Rankine proposed an absolute temperature scale based on the Fahrenheit...
Related questions
Question
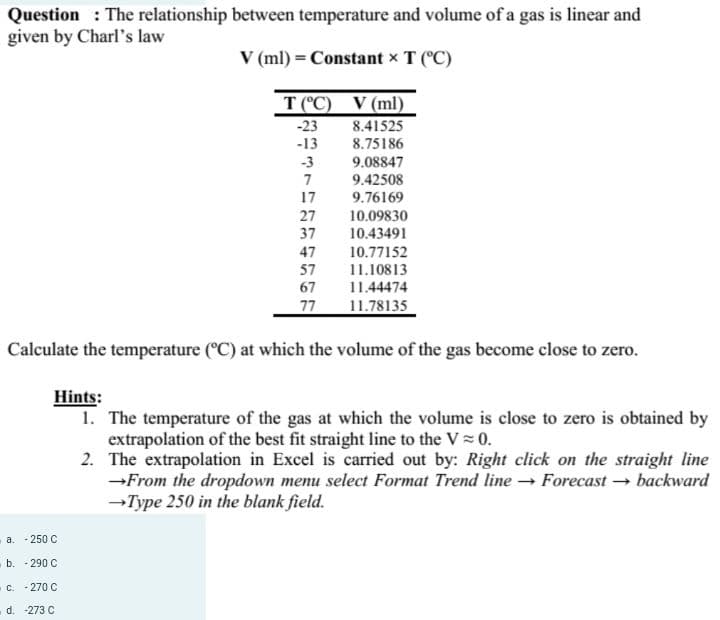
Transcribed Image Text:Question: The relationship between temperature and volume of a gas is linear and
given by Charl's law
V (ml) = Constant x T (°C)
T (°C) V (ml)
-23
8.41525
-13
8.75186
9.08847
9.42508
9.76169
-3
7
17
a. -250 C
b. -290 C
c. -270 C
d. -273 C
27
37
47
57
67
77
10.09830
10.43491
10.77152
11.10813
11.44474
11.78135
Calculate the temperature (°C) at which the volume of the gas become close to zero.
Hints:
1. The temperature of the gas at which the volume is close to zero is obtained by
extrapolation of the best fit straight line to the V≈ 0.
2.
The extrapolation in Excel is carried out by: Right click on the straight line
→From the dropdown menu select Format Trend line → Forecast → backward
→Type 250 in the blank field.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,