The nucleus of an atom accounts for most of the size and mass of the atom. O very little of the size and mass of the atom. O most of the atom's size but very little of its mass. O most of the atom's mass but very little of its size.
The nucleus of an atom accounts for most of the size and mass of the atom. O very little of the size and mass of the atom. O most of the atom's size but very little of its mass. O most of the atom's mass but very little of its size.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 25QAP: Do the proton and the neutron have exactly the same mass? How do the masses of the proton and the...
Related questions
Question
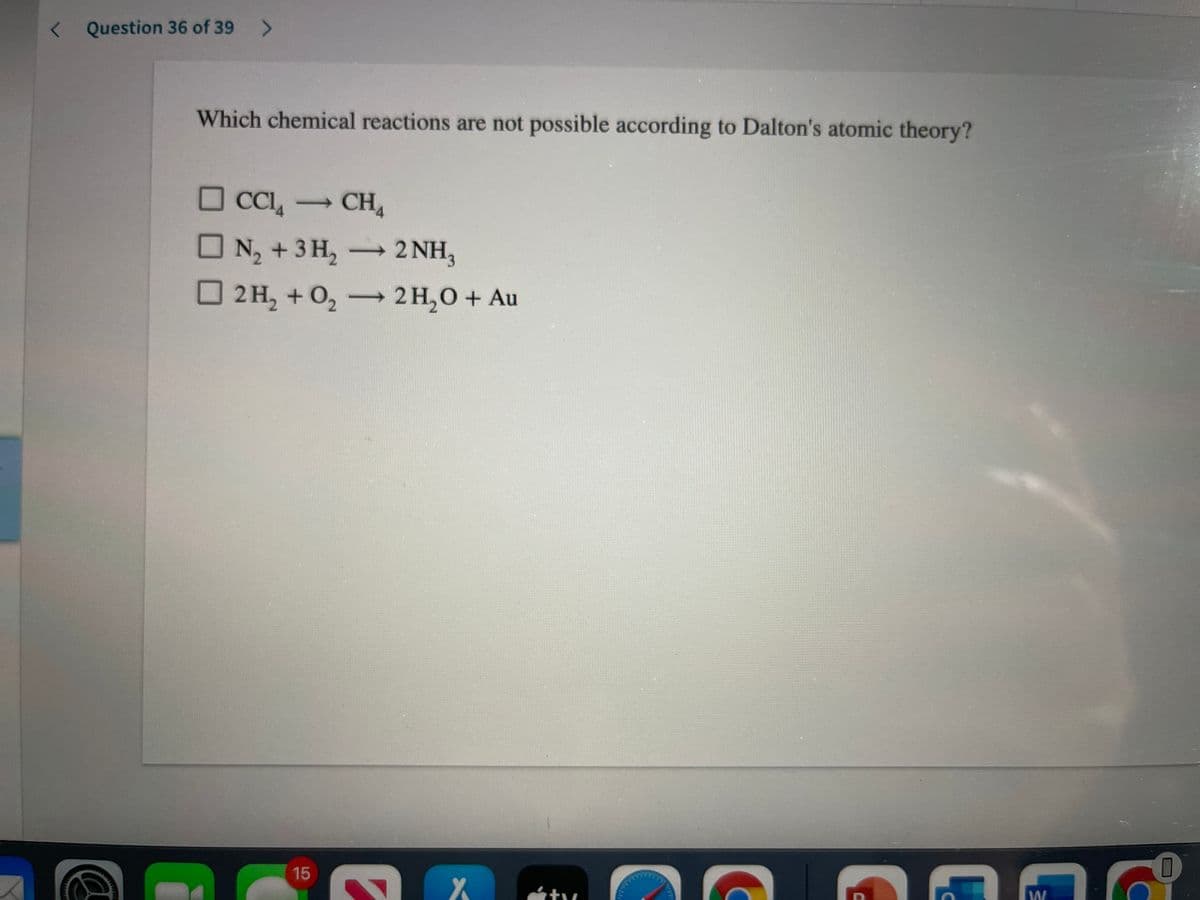
Transcribed Image Text:< Question 36 of 39
Which chemical reactions are not possible according to Dalton's atomic theory?
O CCI, -
→CH4
N2 +3 H, 2NH,
2 H, + 0, —2н,О + Au
15
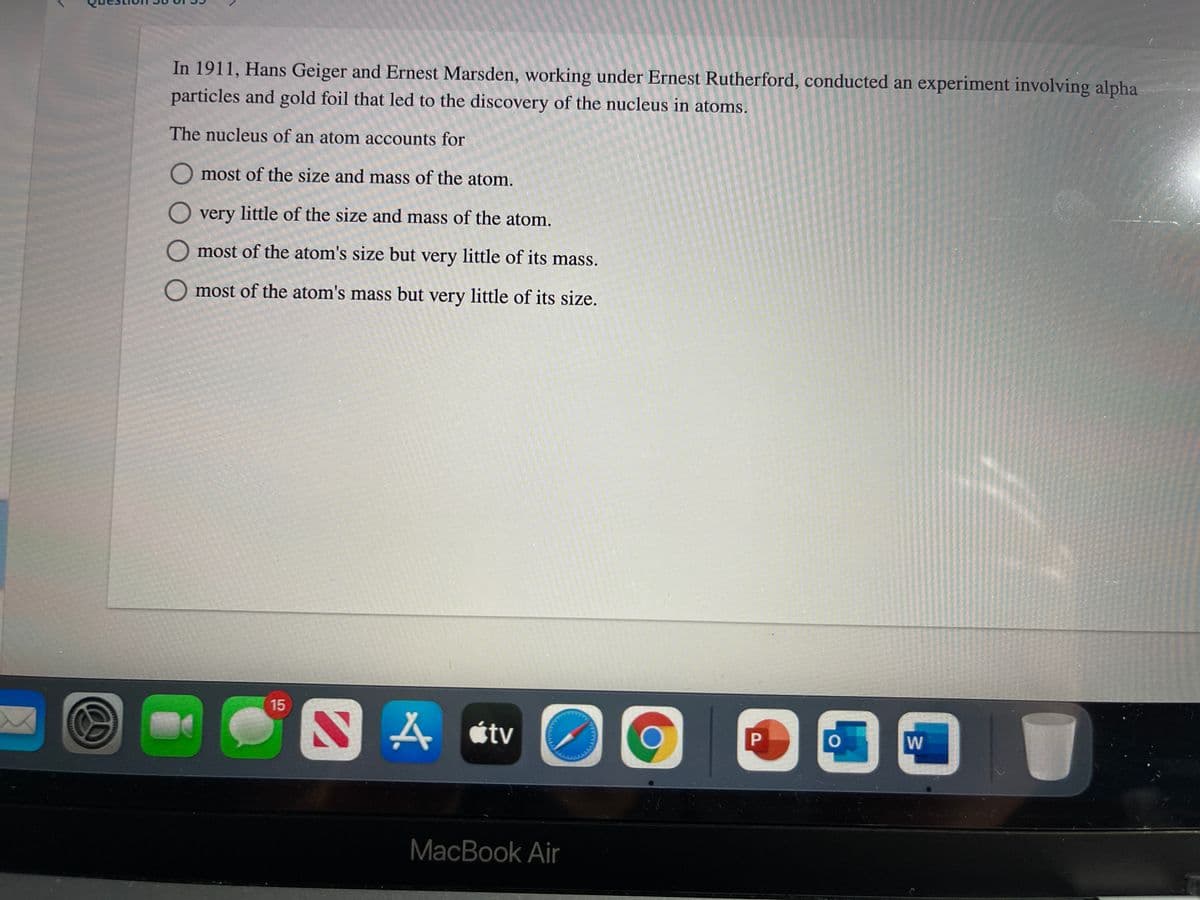
Transcribed Image Text:In 1911, Hans Geiger and Ernest Marsden, working under Ernest Rutherford, conducted an experiment involving alpha
particles and gold foil that led to the discovery of the nucleus in atoms.
The nucleus of an atom accounts for
O most of the size and mass of the atom.
O very little of the size and mass of the atom.
O most of the atom's size but very little of its mass.
O most of the atom's mass but very little of its size.
15
N 4 stv
W
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

