The pH of the human blood is mainly regulated by O a. the phosphate buffering system. O b. a protein buffer system. O . the carbon dioxide - carbonic acid - bicarbonate buffer system. O d. carbonic acid (H2CO3).
The pH of the human blood is mainly regulated by O a. the phosphate buffering system. O b. a protein buffer system. O . the carbon dioxide - carbonic acid - bicarbonate buffer system. O d. carbonic acid (H2CO3).
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter30: Nutrition
Section: Chapter Questions
Problem 30.35P
Related questions
Question
41, 42

Transcribed Image Text:The pH of the human blood is mainly regulated by
the phosphate buffering system.
O b. a protein buffer system.
O c. the carbon dioxide - carbonic acid - bicarbonate buffer system.
O d. carbonic acid (H2CO3).
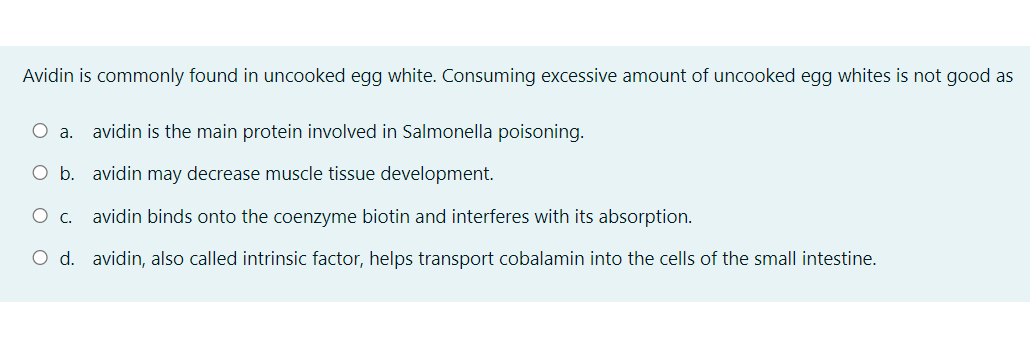
Transcribed Image Text:Avidin is commonly found in uncooked egg white. Consuming excessive amount of uncooked egg whites is not good as
O a.
avidin is the main protein involved in Salmonella poisoning.
O b. avidin may decrease muscle tissue development.
avidin binds onto the coenzyme biotin and interferes with its absorption.
O d. avidin, also called intrinsic factor, helps transport cobalamin into the cells of the small intestine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning