The progress of a reaction is determined by various steps involving energy changes with respect to collision theory. Identify the key features of the reaction profile below. Drag the appropriate labels to their respective targets in the energy profile diagram. • View Available Hint(s) Reset Help ΔΗ Transition State 1 Intermediate Products Transition State Ea2 Reactants Reaction Proeress Energy
The progress of a reaction is determined by various steps involving energy changes with respect to collision theory. Identify the key features of the reaction profile below. Drag the appropriate labels to their respective targets in the energy profile diagram. • View Available Hint(s) Reset Help ΔΗ Transition State 1 Intermediate Products Transition State Ea2 Reactants Reaction Proeress Energy
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 3QAP: How do chemists envision reactions taking place in terms of the collision model for reactions? Give...
Related questions
Question
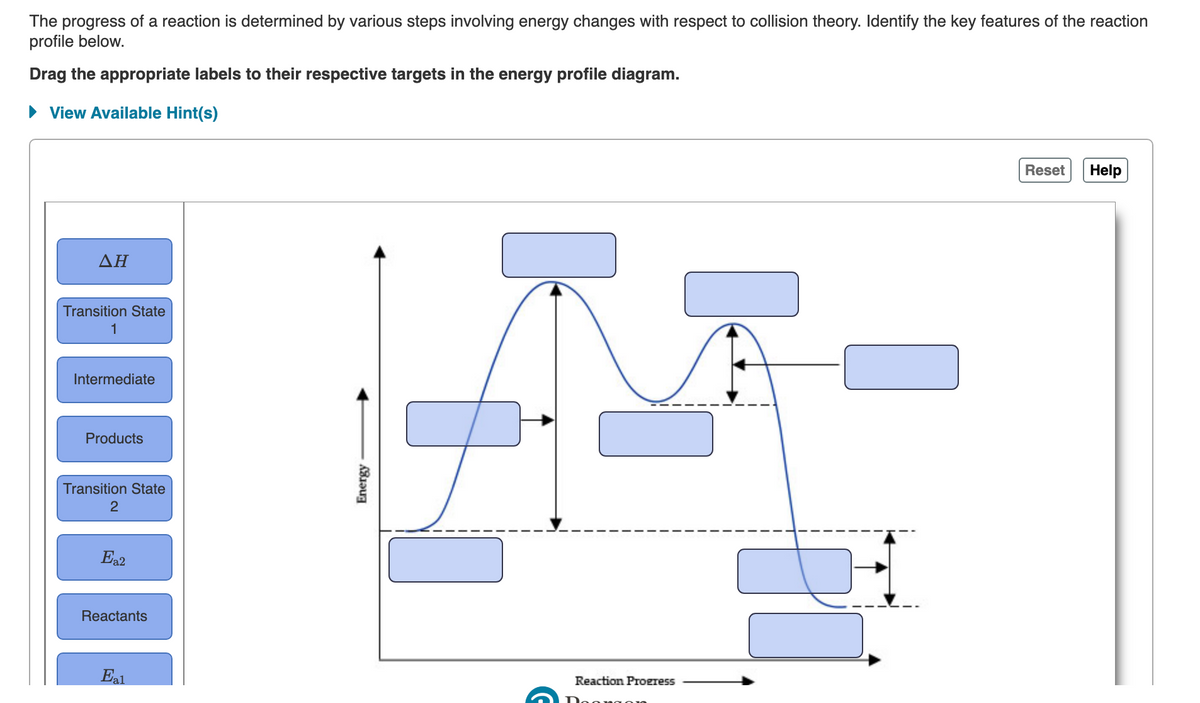
Transcribed Image Text:The progress of a reaction is determined by various steps involving energy changes with respect to collision theory. Identify the key features of the reaction
profile below.
Drag the appropriate labels to their respective targets in the energy profile diagram.
• View Available Hint(s)
Reset
Help
ΔΗ
Transition State
1
Intermediate
Products
Transition State
2
Ea2
Reactants
Eal
Reaction Progress
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning