The reaction: 2 clO2 (aq) → ClO3- (ag) + C1O2¯ (ag) + H2O studied with the following experimental results: Molarity (Na) STOCK (0.09 +2 OH- + CIO2 (ag) + H20 0, was (aq) Final Be Conce Vol (ml) Trial # Rate/M-sec [CIO2/M 0.060 [OH/M 0.030 1 0.0248 0.020 0.030 0.00276 3 0.020 0.090 0.00828 The general expression for the rate law is Rate = k[CIO2]™[OH¯]". Derive the reaction orders, m and n, for the reaction. Show all work. 1. %3D nldn and study the Go to this rinod information provided in the Table(s) above the vialts) smmarized Chemical Bleach Bleach Conc (M) 0.090 Volume (mL) 0.50 0.50 0.180 Yellow Dye 3.40E-05 10.0 Note: there is no "dio"-You s index finger pointing to the bleach be this 2. Use any one(1) of the three trials shown in part 1 to calculate the rate constant k. Show all work. Dil. Bleach tions of Dye, Conc. mixing Blcach the video reactants
The reaction: 2 clO2 (aq) → ClO3- (ag) + C1O2¯ (ag) + H2O studied with the following experimental results: Molarity (Na) STOCK (0.09 +2 OH- + CIO2 (ag) + H20 0, was (aq) Final Be Conce Vol (ml) Trial # Rate/M-sec [CIO2/M 0.060 [OH/M 0.030 1 0.0248 0.020 0.030 0.00276 3 0.020 0.090 0.00828 The general expression for the rate law is Rate = k[CIO2]™[OH¯]". Derive the reaction orders, m and n, for the reaction. Show all work. 1. %3D nldn and study the Go to this rinod information provided in the Table(s) above the vialts) smmarized Chemical Bleach Bleach Conc (M) 0.090 Volume (mL) 0.50 0.50 0.180 Yellow Dye 3.40E-05 10.0 Note: there is no "dio"-You s index finger pointing to the bleach be this 2. Use any one(1) of the three trials shown in part 1 to calculate the rate constant k. Show all work. Dil. Bleach tions of Dye, Conc. mixing Blcach the video reactants
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 7P
Related questions
Question
Please answer the questions on the pictures provided
![The reaction: 2 ClO2 (ag) + 2 OH (aq) → CIO3¯ (aq)
+ ClO2¯ (ag) + H2O a), was
studied with the following experimental results:
Mola
Final
Concentrat
STOCK
(0.
Trial #
[CIO2/M
[OH-]/M
Rate/M-sec-
0.030
0.030
1
0.060
0.0248
0.00276
0.00828
0.020
3.
0.020
0.090
1. The general expression for the rate law is Rate k[CIO2]™[OH ]". Derive the
reaction orders, m and n, for the reaction. Show all work.
and study the
Go to this link
information provided in the Table(s) above the vialfs) smmarized here
Volume (mL)
0.50
Chemical
Conc (M)
Bleach
Bleach
0.090
0.180
0.50
Yellow Dye
Note: there is no "udio"-You t look at his index finger pointing to the bleadh beig ed
3.40E-05
10.0
ble above:
Use any one(1) of the three trials shown in part 1 to calculate the rate constant k. Show
entrations of Dye, Conc. Bleach, and Dil. Bleach
2.
all work.
after mixing the two reactants in the video.
HINT pretend/assume the yellow dye is 100% pure solvent used as a dilucnt
3. Calculate the volumes of stock solution you will need to make 10 mL each of
the dilutions for the following NaOH solutions in Table 2 (on the next page):
(See Part I in the procedure and flow chart provided). One sample calculation
in space below:
page
001M](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F523b9775-526c-4840-92de-ec581e259b38%2F26af96f7-cfc2-4187-ad33-6727cdc106e8%2Fj84glu8_processed.jpeg&w=3840&q=75)
Transcribed Image Text:The reaction: 2 ClO2 (ag) + 2 OH (aq) → CIO3¯ (aq)
+ ClO2¯ (ag) + H2O a), was
studied with the following experimental results:
Mola
Final
Concentrat
STOCK
(0.
Trial #
[CIO2/M
[OH-]/M
Rate/M-sec-
0.030
0.030
1
0.060
0.0248
0.00276
0.00828
0.020
3.
0.020
0.090
1. The general expression for the rate law is Rate k[CIO2]™[OH ]". Derive the
reaction orders, m and n, for the reaction. Show all work.
and study the
Go to this link
information provided in the Table(s) above the vialfs) smmarized here
Volume (mL)
0.50
Chemical
Conc (M)
Bleach
Bleach
0.090
0.180
0.50
Yellow Dye
Note: there is no "udio"-You t look at his index finger pointing to the bleadh beig ed
3.40E-05
10.0
ble above:
Use any one(1) of the three trials shown in part 1 to calculate the rate constant k. Show
entrations of Dye, Conc. Bleach, and Dil. Bleach
2.
all work.
after mixing the two reactants in the video.
HINT pretend/assume the yellow dye is 100% pure solvent used as a dilucnt
3. Calculate the volumes of stock solution you will need to make 10 mL each of
the dilutions for the following NaOH solutions in Table 2 (on the next page):
(See Part I in the procedure and flow chart provided). One sample calculation
in space below:
page
001M
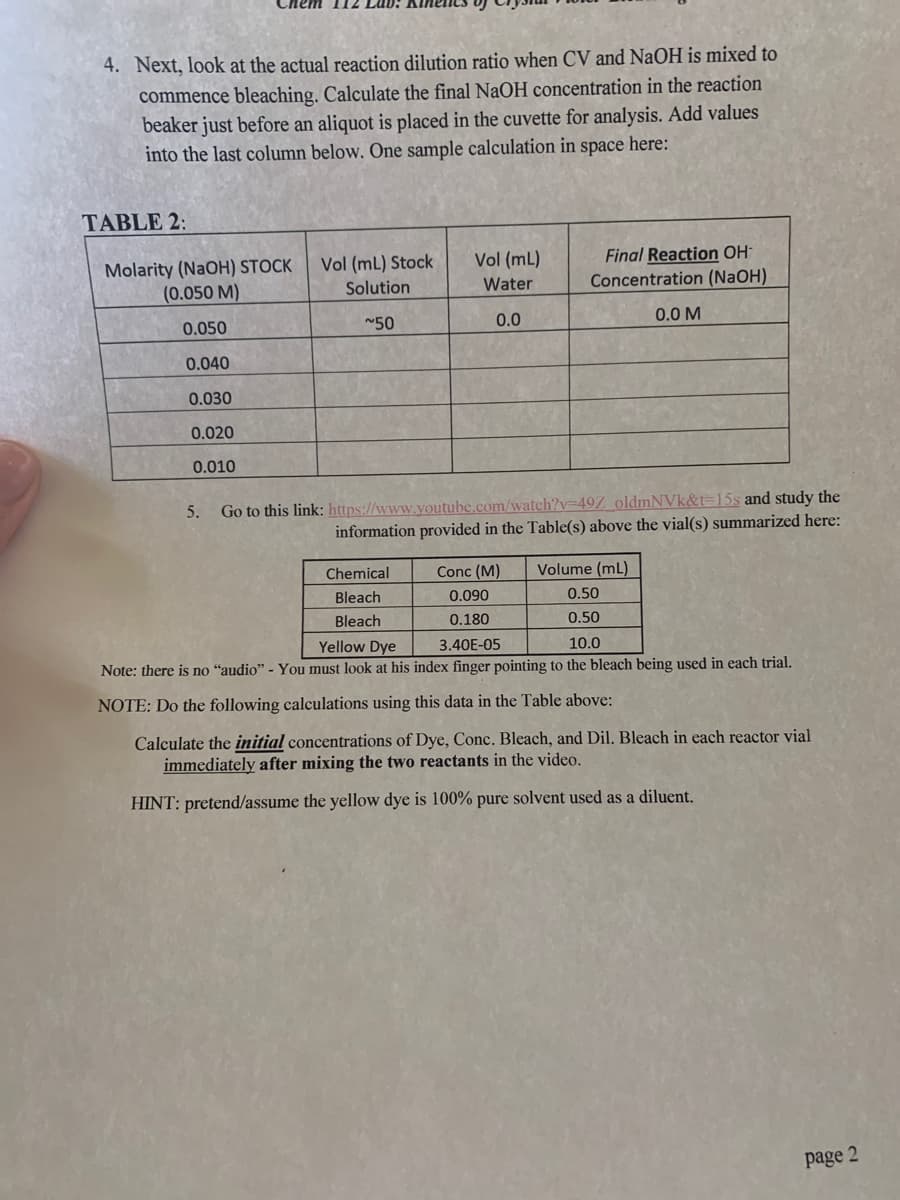
Transcribed Image Text:Chem 112 Lab:
4. Next, look at the actual reaction dilution ratio when CV and NaOH is mixed to
commence bleaching. Calculate the final NaOH concentration in the reaction
beaker just before an aliquot is placed in the cuvette for analysis. Add values
into the last column below. One sample calculation in space here:
TABLE 2:
Final Reaction OH-
Concentration (NaOH)
Vol (mL)
Molarity (NaOH) STOCK
(0.050 M)
Vol (mL) Stock
Solution
Water
0.050
~50
0.0
0.0 M
0.040
0.030
0.020
0.010
Go to this link: https://www.voutube.com/watch?v=49Z_oldmNVk&t=15s and study the
information provided in the Table(s) above the vial(s) summarized here:
5.
Chemical
Conc (M)
Volume (mL)
Bleach
0.090
0.50
Bleach
0.180
0.50
Yellow Dye
3.40E-05
10.0
Note: there is no "audio" - You must look at his index finger pointing to the bleach being used in each trial.
NOTE: Do the following calculations using this data in the Table above:
Calculate the initial concentrations of Dye, Conc. Bleach, and Dil. Bleach in each reactor vial
immediately after mixing the two reactants in the video.
HINT: pretend/assume the yellow dye is 100% pure solvent used as a diluent.
page 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



