The reaction NO (g) + 03 (g) = NO2 (g) + O2 (g) reaches equilibrium at 310 K. Some of the NO2 gas is then removed from the reaction chamber. Which of the followin hat would result when equilibrium is restored? O a. The concentration of O2 decreases. Ob. The equilibrium constant decreases. Oc. The concentration of NO decreases. Od. The concentration of O3 increases. Oe. The concentration of NO remains the same. Moving to another question will save this response. MacBook Air G 80 F3 :6: F2 F1 2 141 #3 F $4 R 70 % 5 S F5 T 6 & Y 7
The reaction NO (g) + 03 (g) = NO2 (g) + O2 (g) reaches equilibrium at 310 K. Some of the NO2 gas is then removed from the reaction chamber. Which of the followin hat would result when equilibrium is restored? O a. The concentration of O2 decreases. Ob. The equilibrium constant decreases. Oc. The concentration of NO decreases. Od. The concentration of O3 increases. Oe. The concentration of NO remains the same. Moving to another question will save this response. MacBook Air G 80 F3 :6: F2 F1 2 141 #3 F $4 R 70 % 5 S F5 T 6 & Y 7
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.103PAE: 12.103 Methanol, CH3OH, can be produced by the reaction of CO with H2, with the liberation of heat....
Related questions
Question
u
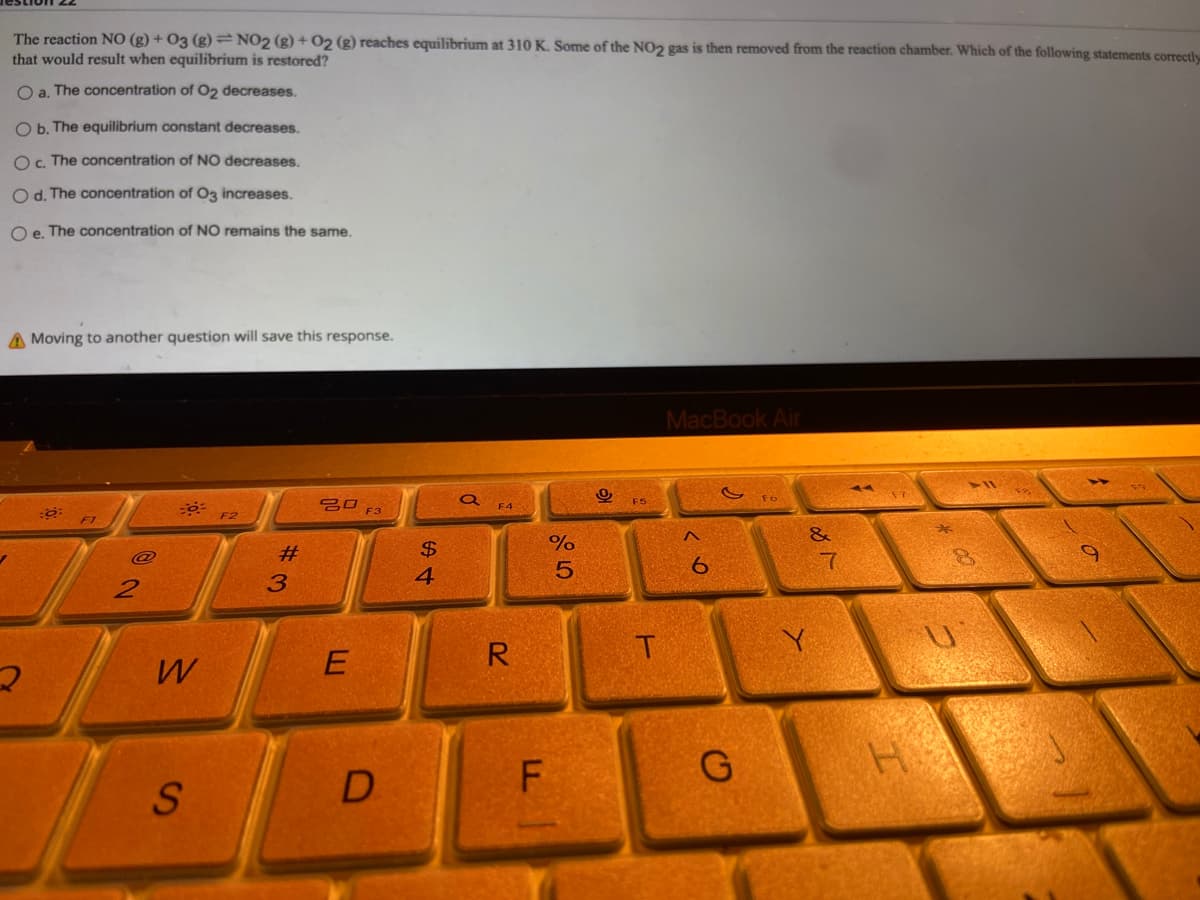
Transcribed Image Text:The reaction NO(g) + 03 (g) = NO2 (g) + O2 (g) reaches equilibrium at 310 K. Some of the NO2 gas is then removed from the reaction chamber. Which of the following statements correctly
that would result when equilibrium is restored?
O a. The concentration of O2 decreases.
O b. The equilibrium constant decreases.
O c. The concentration of NO decreases.
Od. The concentration of O3 increases.
O e. The concentration of NO remains the same.
A Moving to another question will save this response.
MacBook Air
30 F3
30:
O
2
2
W
S
#3
111
E
D
$
54
O
F4
R
70
F
%
5
F5
T
< 6
G
&
Y
7
H
00
o
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning