The Robinson annulation involves two sequential reactions, a Michael addition and an aldol condensation, resulting in the formation of a cyclohexenone derivative. Draw the structure of the two neutral reactants required to form the product shown below. NAOCH, CH,0H
The Robinson annulation involves two sequential reactions, a Michael addition and an aldol condensation, resulting in the formation of a cyclohexenone derivative. Draw the structure of the two neutral reactants required to form the product shown below. NAOCH, CH,0H
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 31MP: The Favorskii reaction involves treatment of an -bromo ketone with base to yield a ring-contracted...
Related questions
Question
100%
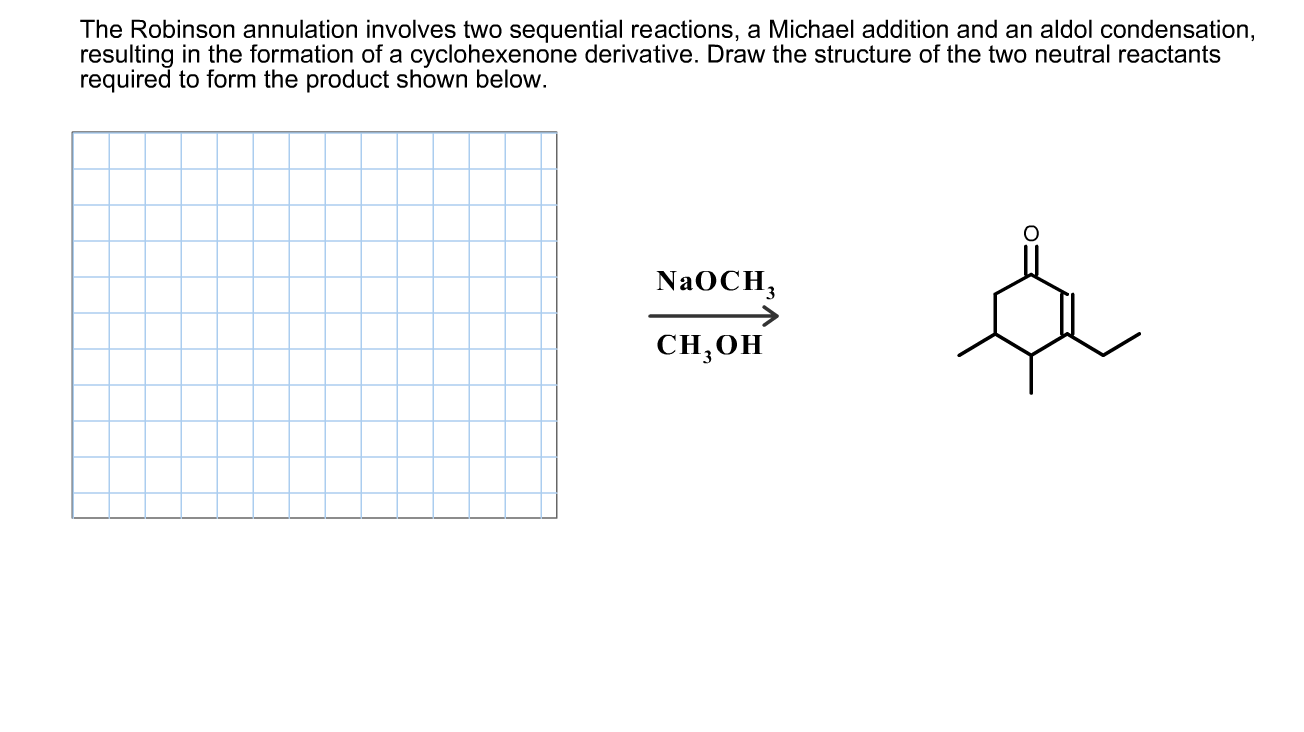
Transcribed Image Text:The Robinson annulation involves two sequential reactions, a Michael addition and an aldol condensation,
resulting in the formation of a cyclohexenone derivative. Draw the structure of the two neutral reactants
required to form the product shown below.
NAOCH,
CH,0H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning