The temperatare dependence of the reaction rate constact is givea by the lak-A- Where - rate conatant T-temperature A- frequency factor E- Energy of activation la order to solve for the Energy of activation, B.you must: S C Add the same expression to each side of the equation to leave the term that includes the variable by itself on the right-hand side of the expression (Be sure that she anwer fleid changes from light yellow to dark yellow before releastng your anwer) x +nk= / +In A- - In A Step Two Multiply both sudes of the equation by the same expressuon (Be sure that the anwer field changes from light yellow to dark yallow before releasing your antwer) x (la k- ln A)- Drag and drop your selection from the fellowing list to complete the answer 1. RT RT RT RT
The temperatare dependence of the reaction rate constact is givea by the lak-A- Where - rate conatant T-temperature A- frequency factor E- Energy of activation la order to solve for the Energy of activation, B.you must: S C Add the same expression to each side of the equation to leave the term that includes the variable by itself on the right-hand side of the expression (Be sure that she anwer fleid changes from light yellow to dark yellow before releastng your anwer) x +nk= / +In A- - In A Step Two Multiply both sudes of the equation by the same expressuon (Be sure that the anwer field changes from light yellow to dark yallow before releasing your antwer) x (la k- ln A)- Drag and drop your selection from the fellowing list to complete the answer 1. RT RT RT RT
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.19QAP
Related questions
Question
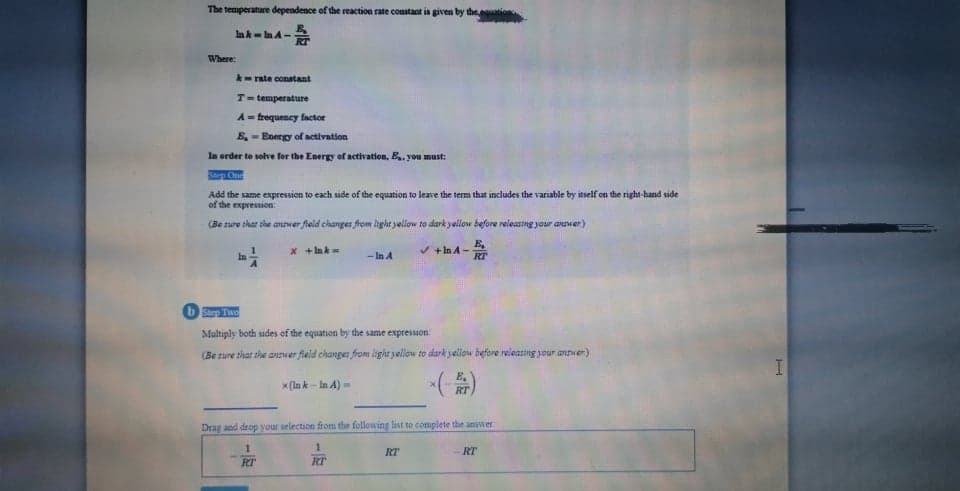
Transcribed Image Text:The temperature dependence of the reaction rate constaot is given by the
In k- n A-
Where:
- rate conatant
T= temperature
A = frequency factor
E, - Energy of activation
In order to solve for the Energy of activation, B.. you must:
Ste Cn
Add the same expression to each side of the equation to leave the term that includes the variable by itself on the right-hand side
of the expression
(Be sure that she anwer field changes from light jellow to dark yellow before releastng your answer)
x + Ink
+In A-
RT
- In A
bStep Two
Multiply both sides of the equation by the same expression
(Be sure that the answer field changes from light yellow to dark yellow before releasting your anver)
x (la k - In A) =
RT
Drag and drop your selection from the following list to complete the anwer
1.
RT
RT
RT
RT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,