The thermogram in Figure below shows the change in mass of a sample of Copper sulfate pentahydrate, CuSO++5H2O (249.5 g/mole) as a function of temperature. The original sample weighing 25.0 mg was heated from room temperature to 1000°C at a rate of 5º C per minute. The following changes in mass and corresponding temperature ranges were observed : Loss of 1.80 mg from 100 – 250° C. Loss of 7.212 mg from 350 – 550° C., Loss of 8.015 mg from 600 – 800° C. Determine the identities of the volatilization products and the solid residue at each step of the thermal decomposition. Given that, H=1 g/mol, S=32 g/mol, O=16 g/mol . 20.00 - 15.00 10.00- 500- 0.00 200 300 400 500 600 700 00 s00 1000 100 Temperature C) Thermogram for CuSO4.5H20 国
The thermogram in Figure below shows the change in mass of a sample of Copper sulfate pentahydrate, CuSO++5H2O (249.5 g/mole) as a function of temperature. The original sample weighing 25.0 mg was heated from room temperature to 1000°C at a rate of 5º C per minute. The following changes in mass and corresponding temperature ranges were observed : Loss of 1.80 mg from 100 – 250° C. Loss of 7.212 mg from 350 – 550° C., Loss of 8.015 mg from 600 – 800° C. Determine the identities of the volatilization products and the solid residue at each step of the thermal decomposition. Given that, H=1 g/mol, S=32 g/mol, O=16 g/mol . 20.00 - 15.00 10.00- 500- 0.00 200 300 400 500 600 700 00 s00 1000 100 Temperature C) Thermogram for CuSO4.5H20 国
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.101QE: In the 1880s, Frederick Trouton noted that the enthalpy of vaporization of 1 mol pure liquid is...
Related questions
Question
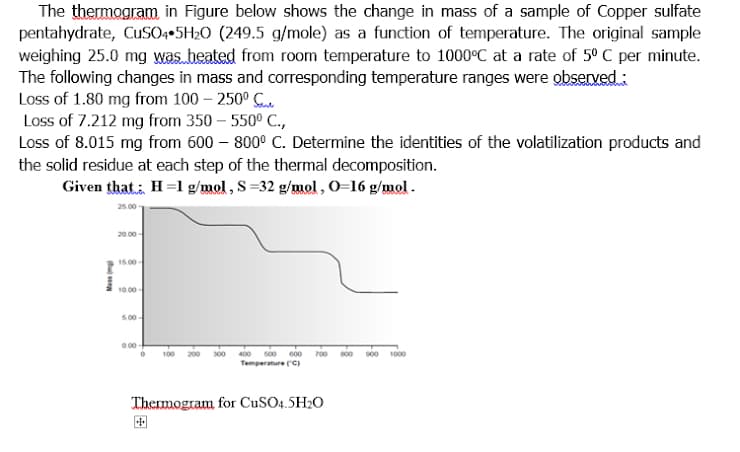
Transcribed Image Text:The thermagram in Figure below shows the change in mass of a sample of Copper sulfate
pentahydrate, CuSO++5H2O (249.5 g/mole) as a function of temperature. The original sample
weighing 25.0 mg was heated from room temperature to 1000°C at a rate of 5º C per minute.
The following changes in mass and corresponding temperature ranges were observed :
Loss of 1.80 mg from 100 – 250° C.
Loss of 7.212 mg from 350 – 550° C.,
Loss of 8.015 mg from 600 – 800° C. Determine the identities of the volatilization products and
the solid residue at each step of the thermal decomposition.
Given that: H=1 g/mol , S =32 g/mol , 0=16 g/mol.
20.00 -
15.00
10.00
500-
0.00
400
s00 600 700
Temperature C)
100
200 300
00 s00 1000
Thermogram for CUSO4.5H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning