Part A The metabolism of glucose, CH1206. yields carbon dioxide, CO2(g), and water, H2O(1), as products. Energy released in this metabolic process is converted to useful work, w, with about 67.0 % efficiency. The formula for work is Calculate the mass of glucose metabolized by a 83.0 kg person in climbing a mountain with an elevation gain of 1470 m. Assume that the work performed in the climb is four times that required to simply lift 83.0 kg by 1470 m. w = mdg Express your answer to three significant figures and include the appropriate units. where m is mass, d is distance, and g=9.81 m -s2 is the acceleration due to gravity. > View Available Hint(s) Use the data below to answer questions about the metabolism of glucose. ? ΔΗ (kJ/mol) Substance Value Units CO2 (g) -393.5 C,H12O6(s) -1273.3 Submit H2O(1) -285.8 O2(g)
Part A The metabolism of glucose, CH1206. yields carbon dioxide, CO2(g), and water, H2O(1), as products. Energy released in this metabolic process is converted to useful work, w, with about 67.0 % efficiency. The formula for work is Calculate the mass of glucose metabolized by a 83.0 kg person in climbing a mountain with an elevation gain of 1470 m. Assume that the work performed in the climb is four times that required to simply lift 83.0 kg by 1470 m. w = mdg Express your answer to three significant figures and include the appropriate units. where m is mass, d is distance, and g=9.81 m -s2 is the acceleration due to gravity. > View Available Hint(s) Use the data below to answer questions about the metabolism of glucose. ? ΔΗ (kJ/mol) Substance Value Units CO2 (g) -393.5 C,H12O6(s) -1273.3 Submit H2O(1) -285.8 O2(g)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 12QAP: The heat of neutralization, Hneut, can be defined as the amount of heat released (or absorbed), q,...
Related questions
Question
Chemistry
Please kindly answer this question, thank you in advance!!!!
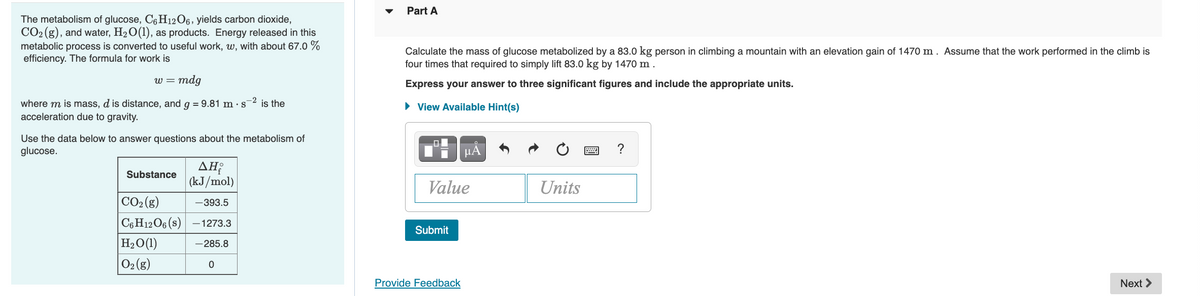
Transcribed Image Text:Part A
The metabolism of glucose, C6 H12O6, yields carbon dioxide,
CO2 (g), and water, H2O(1), as products. Energy released in this
metabolic process is converted to useful work, w, with about 67.0 %
efficiency. The formula for work is
Calculate the mass of glucose metabolized by a 83.0 kg person in climbing a mountain with an elevation gain of 1470 m. Assume that the work performed in the climb is
four times that required to simply lift 83.0 kg by 1470 m .
W =
mdg
Express your answer to three significant figures and include the appropriate units.
where m is mass, d is distance, and g = 9.81 m · s2 is the
acceleration due to gravity.
• View Available Hint(s)
Use the data below to answer questions about the metabolism of
glucose.
µA
ΔΗ
(kJ/mol)
Substance
Value
Units
CO2 (g)
-393.5
C6 H12O6 (s) -1273.3
Submit
H2O(1)
O2 (g)
-285.8
Provide Feedback
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning