The two square-planar isomers of [PtBrCl(PR3)2] (where PR3is a trialkylphosphene) have different 31P-NMR spectra as shown below. For this exercise, we ignore the coupling to 195Pt (I=1/2 at 33% abundance). One isomer (A) shows a single 31P resonance; the other (B) shows two 31P resonances, each of which is split into a doublet by the second 31P nucleus. Which isomer is cisand which is trans? Draw the structures of the two isomers.
The two square-planar isomers of [PtBrCl(PR3)2] (where PR3is a trialkylphosphene) have different 31P-NMR spectra as shown below. For this exercise, we ignore the coupling to 195Pt (I=1/2 at 33% abundance). One isomer (A) shows a single 31P resonance; the other (B) shows two 31P resonances, each of which is split into a doublet by the second 31P nucleus. Which isomer is cisand which is trans? Draw the structures of the two isomers.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 5A
Related questions
Question
100%
The two square-planar isomers of [PtBrCl(PR3)2] (where PR3is a trialkylphosphene) have different 31P-NMR spectra as shown below. For this exercise, we ignore the coupling to 195Pt (I=1/2 at 33% abundance). One isomer (A) shows a single 31P resonance; the other (B) shows two 31P resonances, each of which is split into a doublet by the second 31P nucleus. Which isomer is cisand which is trans? Draw the structures of the two isomers.
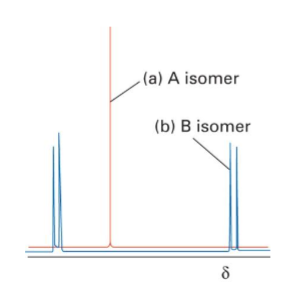
Transcribed Image Text:-(a) A isomer
(b) B isomer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co