The U.S. Environmental Protection Agency (U.S. EPA) monitors air quality in the United States. Lead is among the pollutants regularly monitored and regulated. Lead is released into the at- mosphere primarily by the processing of metals ores containing lead and by lead-based battery manufacturing. The effects of too much exposure to lead include neurological damage and cardio- vascular disease. Because of the Clean Air Act and its amend- ments, the amounts of lead in air have been decreasing for many years. The chart below shows the lead concentration in air in the United States from 2000 to 2014. Examine the data and answer the questions below Lead Concentration in Air in U.S. 0.35 0.30 0.25 0.20 0.15 0.10 E 0.05 1998 2000 2002 2004 2006 2008 2010 2012 2014 2016 Year 100% 50% - 204 206 207 208 Mass (amu) Calculate the percent change in lead concentration that occurred from 2000 to 2014. Hint: Calculate the percent change with the following equation: (final concentration – initial concentration) percent change x 100% initial concentration Pb Concentration ug/m Intensity %
The U.S. Environmental Protection Agency (U.S. EPA) monitors air quality in the United States. Lead is among the pollutants regularly monitored and regulated. Lead is released into the at- mosphere primarily by the processing of metals ores containing lead and by lead-based battery manufacturing. The effects of too much exposure to lead include neurological damage and cardio- vascular disease. Because of the Clean Air Act and its amend- ments, the amounts of lead in air have been decreasing for many years. The chart below shows the lead concentration in air in the United States from 2000 to 2014. Examine the data and answer the questions below Lead Concentration in Air in U.S. 0.35 0.30 0.25 0.20 0.15 0.10 E 0.05 1998 2000 2002 2004 2006 2008 2010 2012 2014 2016 Year 100% 50% - 204 206 207 208 Mass (amu) Calculate the percent change in lead concentration that occurred from 2000 to 2014. Hint: Calculate the percent change with the following equation: (final concentration – initial concentration) percent change x 100% initial concentration Pb Concentration ug/m Intensity %
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 130E
Related questions
Question
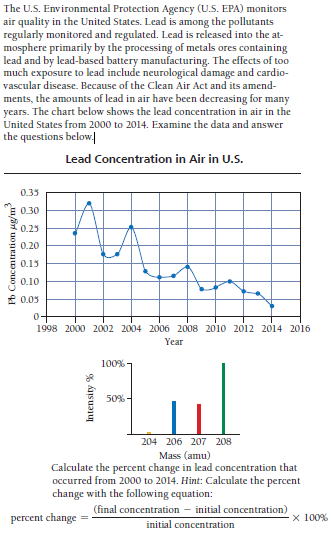
Transcribed Image Text:The U.S. Environmental Protection Agency (U.S. EPA) monitors
air quality in the United States. Lead is among the pollutants
regularly monitored and regulated. Lead is released into the at-
mosphere primarily by the processing of metals ores containing
lead and by lead-based battery manufacturing. The effects of too
much exposure to lead include neurological damage and cardio-
vascular disease. Because of the Clean Air Act and its amend-
ments, the amounts of lead in air have been decreasing for many
years. The chart below shows the lead concentration in air in the
United States from 2000 to 2014. Examine the data and answer
the questions below
Lead Concentration in Air in U.S.
0.35
0.30
0.25
0.20
0.15
0.10
E 0.05
1998 2000 2002 2004 2006 2008 2010 2012 2014 2016
Year
100%
50% -
204 206 207 208
Mass (amu)
Calculate the percent change in lead concentration that
occurred from 2000 to 2014. Hint: Calculate the percent
change with the following equation:
(final concentration – initial concentration)
percent change
x 100%
initial concentration
Pb Concentration ug/m
Intensity %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning