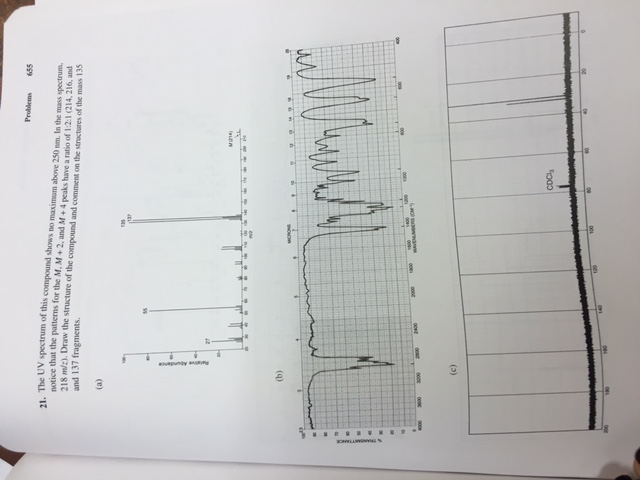
The UV spectrum of this compound shows no maximum above 250 nm. In the mass spectrum, notice that the patterns for the M, M+2, and M+4 peaks have a ratio of 1:2:1 (2*Br) at 214, 216, and 218 m/z. Draw the compound and comment on the structures of the mass 135 and 137 fragments.
Analyzing Infrared Spectra
The electromagnetic radiation or frequency is classified into radio-waves, micro-waves, infrared, visible, ultraviolet, X-rays and gamma rays. The infrared spectra emission refers to the portion between the visible and the microwave areas of electromagnetic spectrum. This spectral area is usually divided into three parts, near infrared (14,290 – 4000 cm-1), mid infrared (4000 – 400 cm-1), and far infrared (700 – 200 cm-1), respectively. The number set is the number of the wave (cm-1).
IR Spectrum Of Cyclohexanone
It is the analysis of the structure of cyclohexaone using IR data interpretation.
IR Spectrum Of Anisole
Interpretation of anisole using IR spectrum obtained from IR analysis.
IR Spectroscopy
Infrared (IR) or vibrational spectroscopy is a method used for analyzing the particle's vibratory transformations. This is one of the very popular spectroscopic approaches employed by inorganic as well as organic laboratories because it is helpful in evaluating and distinguishing the frameworks of the molecules. The infra-red spectroscopy process or procedure is carried out using a tool called an infrared spectrometer to obtain an infrared spectral (or spectrophotometer).
The UV spectrum of this compound shows no maximum above 250 nm. In the mass spectrum, notice that the patterns for the M, M+2, and M+4 peaks have a ratio of 1:2:1 (2*Br) at 214, 216, and 218 m/z. Draw the compound and comment on the structures of the mass 135 and 137 fragments.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images




