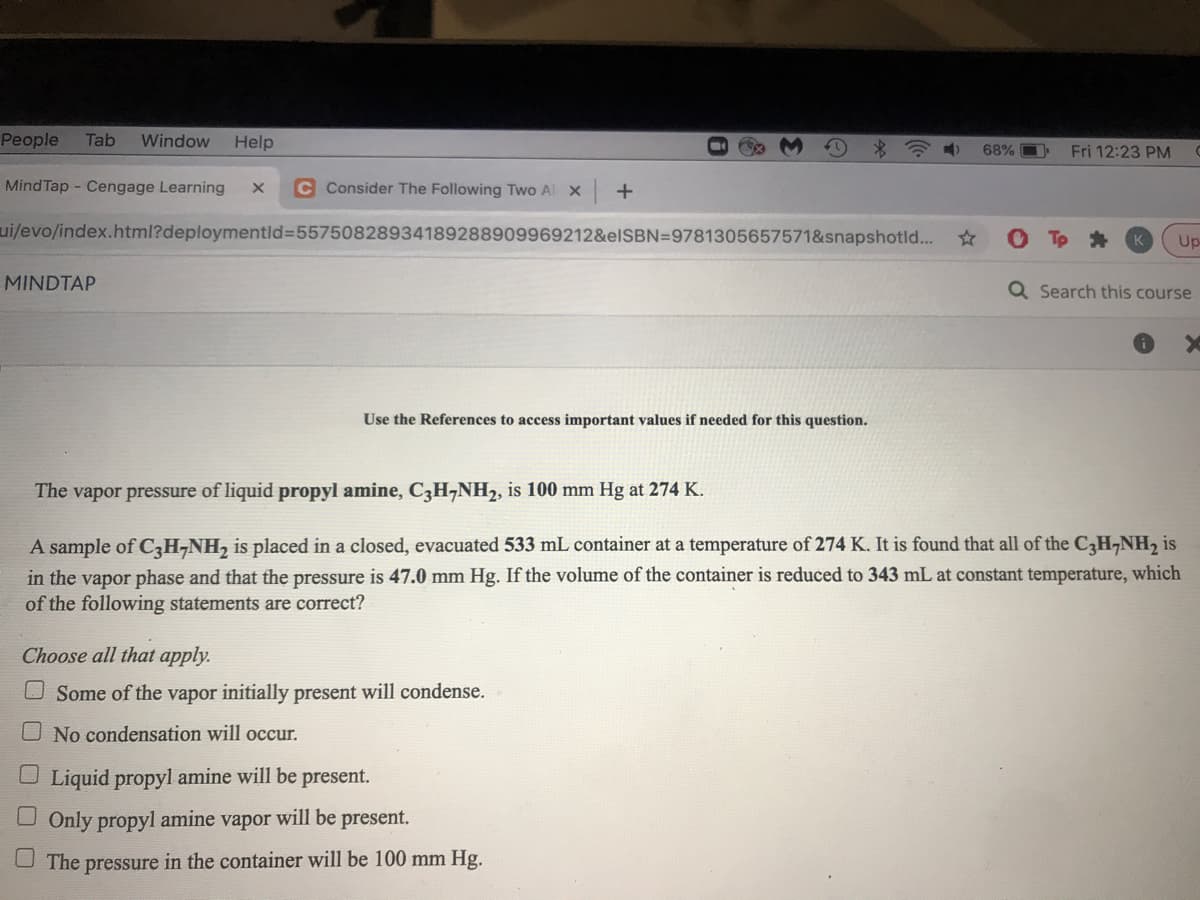
The vapor pressure of liquid propyl amine, C3H¬NH2, is 100 mm Hg at 274 K. A sample of C3H¬NH2 is placed in a closed, evacuated 533 mL container at a temperature of 274 K. It is found that all of the CH¬NH, is in the vapor phase and that the pressure is 47.0 mm Hg. If the volume of the container is reduced to 343 mL at constant temperature, which of the following statements are correct? Choose all that apply. U Some of the vapor initially present will condense. O No condensation will occur. Liquid propyl amine will be present. O Only propyl amine vapor will be present. The pressure in the container will be 100 mm Hg.
The vapor pressure of liquid propyl amine, C3H¬NH2, is 100 mm Hg at 274 K. A sample of C3H¬NH2 is placed in a closed, evacuated 533 mL container at a temperature of 274 K. It is found that all of the CH¬NH, is in the vapor phase and that the pressure is 47.0 mm Hg. If the volume of the container is reduced to 343 mL at constant temperature, which of the following statements are correct? Choose all that apply. U Some of the vapor initially present will condense. O No condensation will occur. Liquid propyl amine will be present. O Only propyl amine vapor will be present. The pressure in the container will be 100 mm Hg.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 16QAP: Computers are not supposed to be in very warm rooms. The highest temperature tolerated for maximum...
Related questions
Question
Transcribed Image Text:People
Tab
Window
Help
68% D
Fri 12:23 PM
Mind Tap - Cengage Learning
C Consider The Following Two Al X
ui/evo/index.html?deploymentld3D55750828934189288909969212&elSBN=9781305657571&snapshotld...
Up
MINDTAP
Q Search this course
Use the References to access important values if needed for this question.
The vapor pressure of liquid propyl amine, C3H¬NH2, is 100 mm Hg at 274 K.
A sample of C3H¬NH, is placed in a closed, evacuated 533 mL container at a temperature of 274 K. It is found that all of the CH,NH, is
in the vapor phase and that the pressure is 47.0 mm Hg. If the volume of the container is reduced to 343 mL at constant temperature, which
of the following statements are correct?
Choose all that apply.
O Some of the vapor initially present will condense.
No condensation will occur.
O Liquid propyl amine will be present.
U Only propyl amine vapor will be present.
The pressure in the container will be 100 mm Hg.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning