They are similar to covalent in that electrons are shared in the electron se They are similar to ionic in that the metal atoms gain electrons They are similar to ionic in that the metal atoms lose electrons The electron sea gives metals the property of being bendable
They are similar to covalent in that electrons are shared in the electron se They are similar to ionic in that the metal atoms gain electrons They are similar to ionic in that the metal atoms lose electrons The electron sea gives metals the property of being bendable
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 2STP
Related questions
Question
1. Match the type of bond to the description
2. Which of the following statements are true of metallic bonds?

Transcribed Image Text:Which of the following statements are true of metallic bonds?
O They are similar to covalent in that electrons are shared in the electron sea
They are similar to ionic in that the metal atoms gain electrons
O They are similar to ionic in that the metal atoms lose electrons
The electron sea gives metals the property of being bendable
O The electron sea causes metals to be very poor conductors of electricity
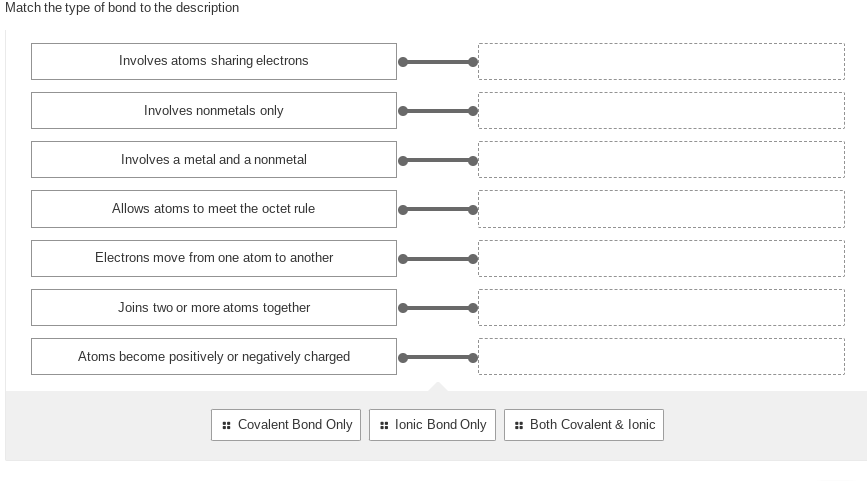
Transcribed Image Text:Match the type of bond to the description
Involves atoms sharing electrons
Involves nonmetals only
Involves a metal and a nonmetal
Allows atoms to meet the octet rule
Electrons move from one atom to another
Joins two or more atoms together
Atoms become positively or negatively charged
:: Covalent Bond Only
I II II
::lonic Bond Only
:: Both Covalent & lonic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax