This graph shows how the vapor pressure of three liquids varies with temperature: 900 800- 700-> 600T 500 400 300- 200 - isobutyl alcohol ethylbenzene 100- -octane 100 1i0 120 130 140 temperature, °C Put these liquids (the names of the liquid) in order from most volatile to least volatile. Most volatile 1 E isobutyl alcohol | octane 3 I ethylbenzene 2. vapor pressure, torr
This graph shows how the vapor pressure of three liquids varies with temperature: 900 800- 700-> 600T 500 400 300- 200 - isobutyl alcohol ethylbenzene 100- -octane 100 1i0 120 130 140 temperature, °C Put these liquids (the names of the liquid) in order from most volatile to least volatile. Most volatile 1 E isobutyl alcohol | octane 3 I ethylbenzene 2. vapor pressure, torr
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.97QE: Sketch graphs of total vapor pressure versus the mole fraction of two volatile substances that show...
Related questions
Question
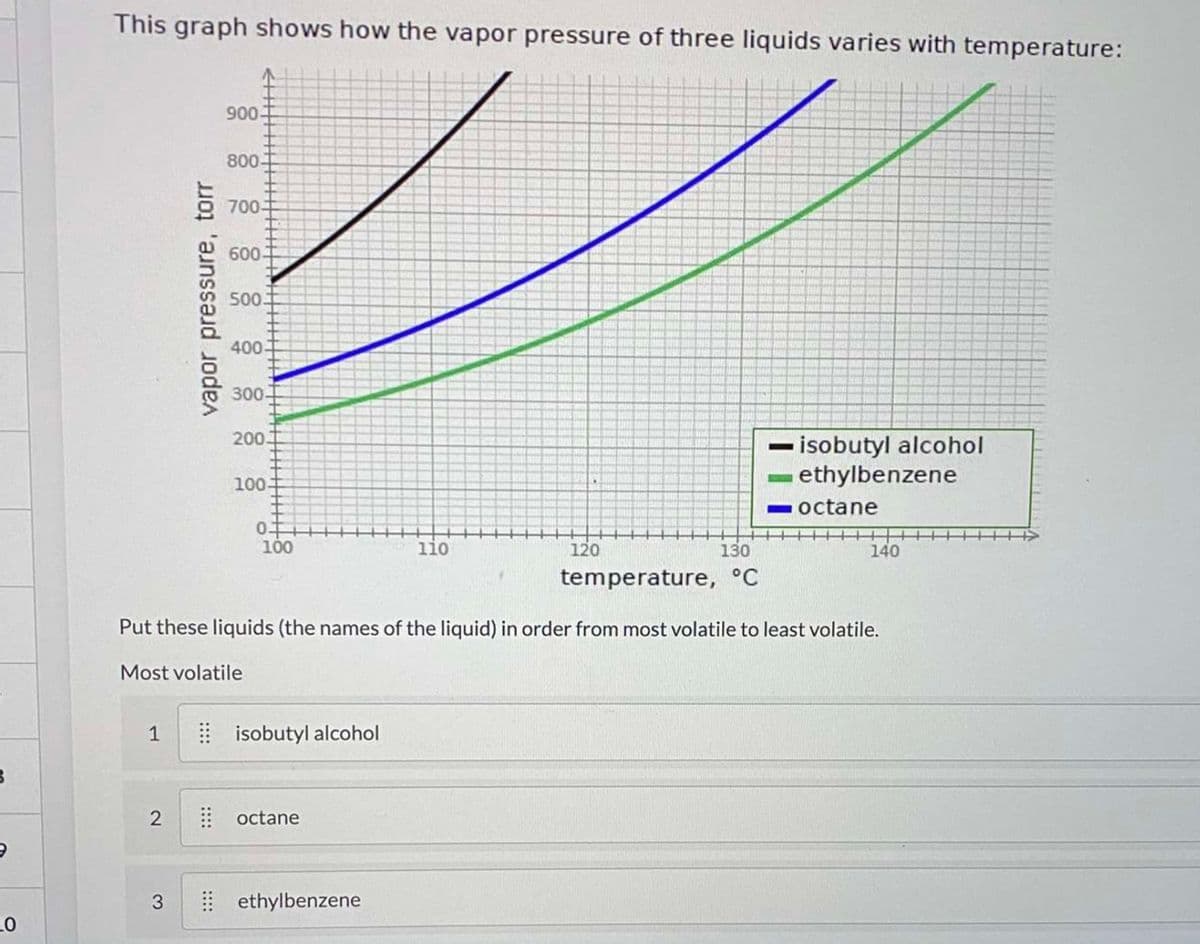
Transcribed Image Text:This graph shows how the vapor pressure of three liquids varies with temperature:
900
800-
700-
600-
50.
400-
300-
200.
- isobutyl alcohol
ethylbenzene
100
-octane
100
110
120
130
140
temperature, °C
Put these liquids (the names of the liquid) in order from most volatile to least volatile.
Most volatile
1
E isobutyl alcohol
2
octane
3
| ethylbenzene
10
vapor pressure, torr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,