Three samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -5.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 2.0 1.9 1.3 volume (L) 10.0 15.0 20.0 ideal? yes O no O yes Ono O yes O no If not ideal, the most important reason why not: There are attractions between the particles. The particles don't have zero volume. There are attractions between the particles. The particles don't have zero volume. There are attractions between the particles. The particles don't have zero volume.
Three samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -5.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 2.0 1.9 1.3 volume (L) 10.0 15.0 20.0 ideal? yes O no O yes Ono O yes O no If not ideal, the most important reason why not: There are attractions between the particles. The particles don't have zero volume. There are attractions between the particles. The particles don't have zero volume. There are attractions between the particles. The particles don't have zero volume.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 7STP
Related questions
Question
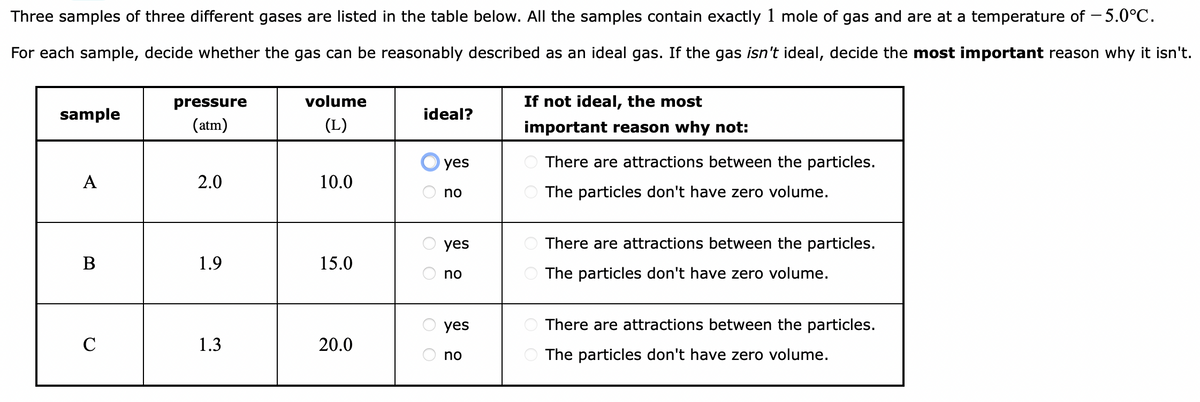
Transcribed Image Text:Three samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -5.0°C.
For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't.
sample
A
B
C
pressure
(atm)
2.0
1.9
1.3
volume
(L)
10.0
15.0
20.0
ideal?
оо
yes
no
yes
no
yes
no
If not ideal, the most
important reason why not:
There are attractions between the particles.
The particles don't have zero volume.
There are attractions between the particles.
The particles don't have zero volume.
There are attractions between the particles.
The particles don't have zero volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning