To a blank graph template, please draw a graph that displays the distribution of velocities of Neon atoms in an isolated system at 300K. Label or annotate to indicate temperature. To this same graph, please draw a graph that displays the distribution of velocities of Neon atoms in an isolated system at 400K. Label or annotate to indicate temperature. In the box below, describe the difference in these two distributions. Upload your drawing at the end of the assignment. Use the editor to format your answer
To a blank graph template, please draw a graph that displays the distribution of velocities of Neon atoms in an isolated system at 300K. Label or annotate to indicate temperature. To this same graph, please draw a graph that displays the distribution of velocities of Neon atoms in an isolated system at 400K. Label or annotate to indicate temperature. In the box below, describe the difference in these two distributions. Upload your drawing at the end of the assignment. Use the editor to format your answer
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter16: Introduction To Magnetic Spectroscopy
Section: Chapter Questions
Problem 16.1E
Related questions
Question
Transcribed Image Text:Velocity
Number of Atoms
:
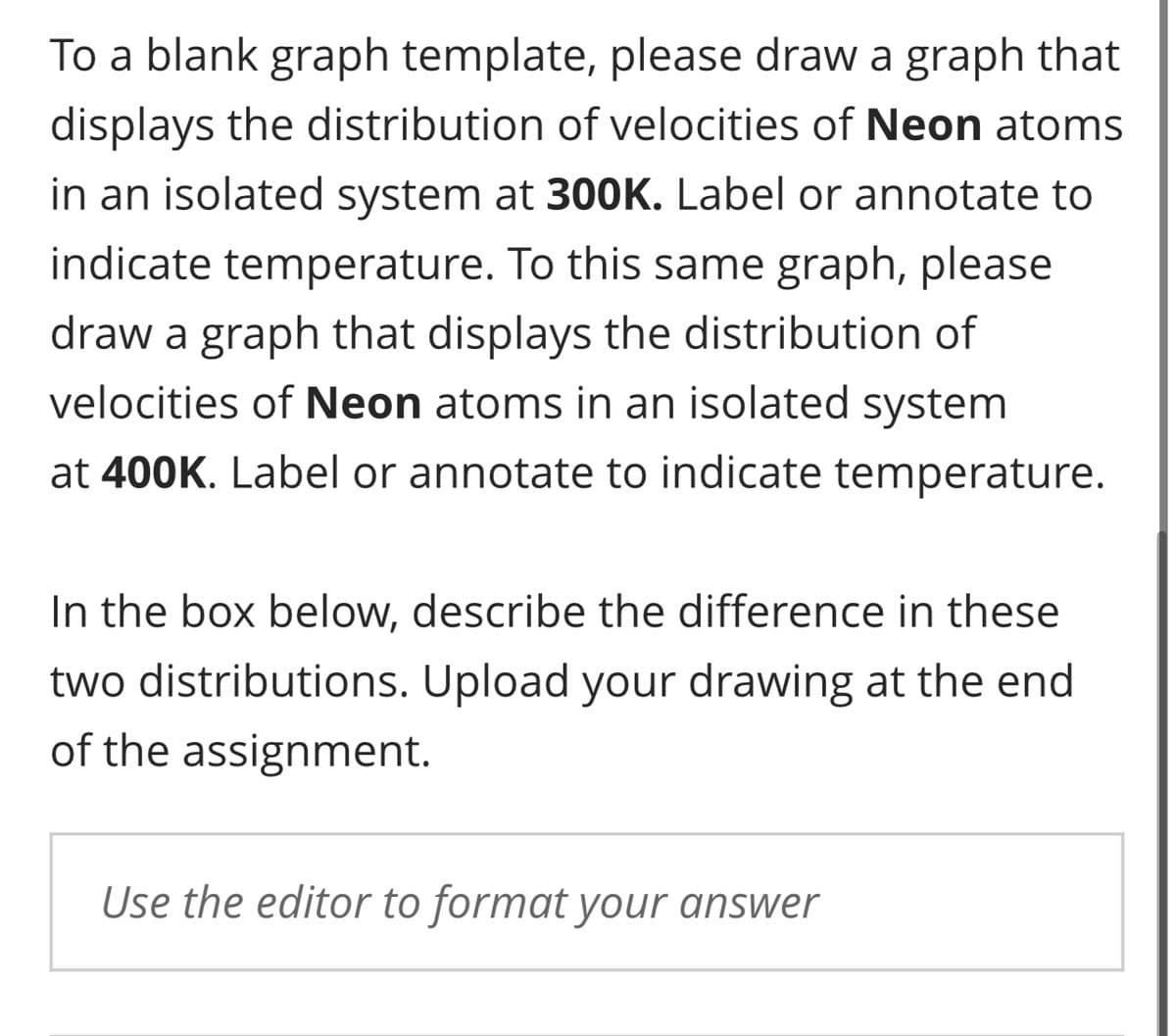
Transcribed Image Text:To a blank graph template, please draw a graph that
displays the distribution of velocities of Neon atoms
in an isolated system at 300K. Label or annotate to
indicate temperature. To this same graph, please
draw a graph that displays the distribution of
velocities of Neon atoms in an isolated system
at 400K. Label or annotate to indicate temperature.
In the box below, describe the difference in these
two distributions. Upload your drawing at the end
of the assignment.
Use the editor to format your answer
Expert Solution
Step 1
The Maxwell-Boltzmann distribution of velocity gives the idea about the what fraction of molecules are moving in (V+dV) velocity range.
It is based on the motion of molecule and kinetic energy of molecules.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning