To determine the experimental heat of combustion of an alcohol, the set-up was assembled as shown below. Experimental results and standard constants were tabulated: Thermometer Draught shield Experimental Data: Initial mass of spirit burner with alcohol 224.59 grams 223.42 grams 26.3°C Copper calorimeter Final mass of spirit burner with alcohol Initial temperature of water in calorimeter before heating Final temperature of water in calorimeter after heating Clamp 56.5 °C Water Standard Constants: (250 ml) 4.184 Joule/gram°C Specific heat of water Molar mass of the alcohol used 46 gram per mole -1368 kJoule per mole Spirit burner Theoretical heat of combustion of the alcohol Liquid fuel Express how close or how far the experimental value for the heat of combustion is to the theoretical expected value. P Type here to search
To determine the experimental heat of combustion of an alcohol, the set-up was assembled as shown below. Experimental results and standard constants were tabulated: Thermometer Draught shield Experimental Data: Initial mass of spirit burner with alcohol 224.59 grams 223.42 grams 26.3°C Copper calorimeter Final mass of spirit burner with alcohol Initial temperature of water in calorimeter before heating Final temperature of water in calorimeter after heating Clamp 56.5 °C Water Standard Constants: (250 ml) 4.184 Joule/gram°C Specific heat of water Molar mass of the alcohol used 46 gram per mole -1368 kJoule per mole Spirit burner Theoretical heat of combustion of the alcohol Liquid fuel Express how close or how far the experimental value for the heat of combustion is to the theoretical expected value. P Type here to search
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter15: Energy And Chemical Change
Section: Chapter Questions
Problem 112A: sample of natural gas is analyzed and found to be88.4% methane (CH4) and 11.6% ethane (C2H6) bymass....
Related questions
Question
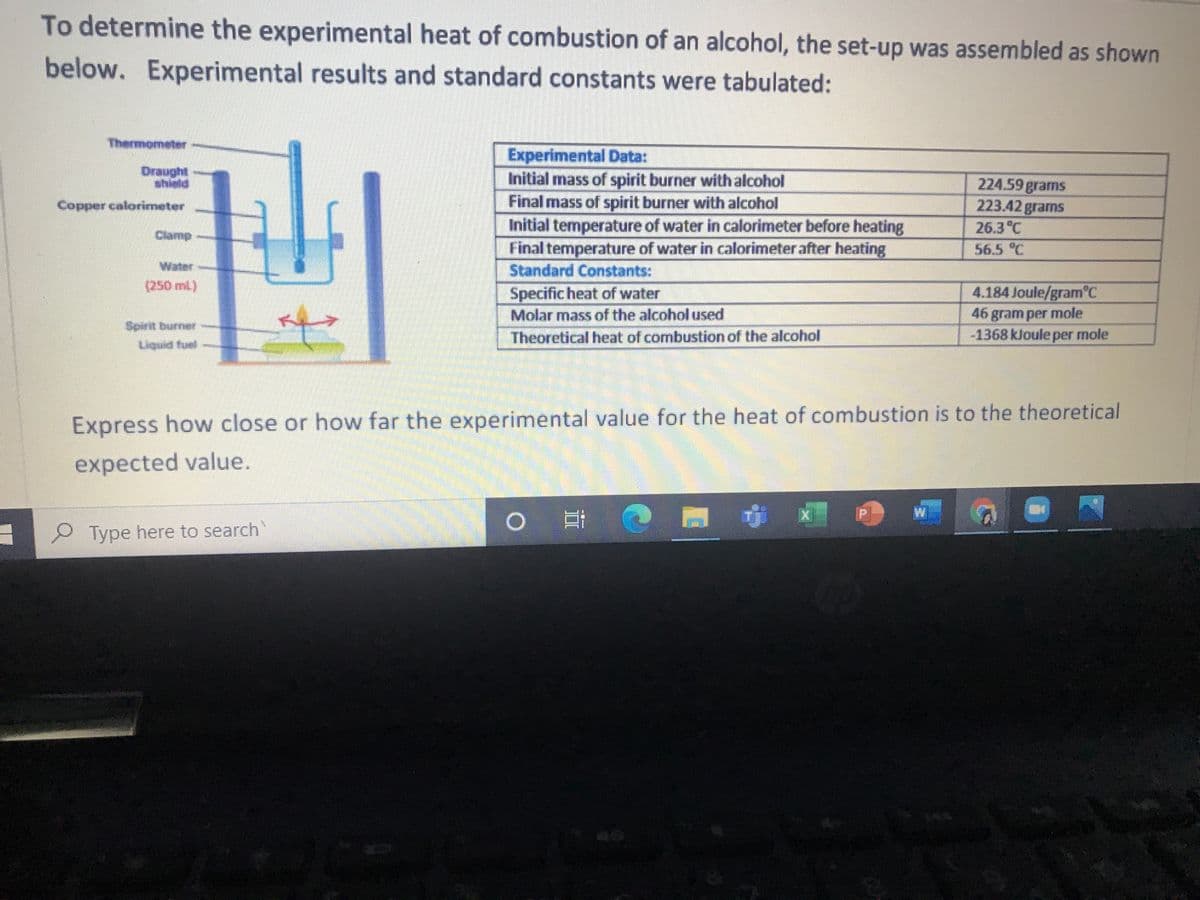
Transcribed Image Text:To determine the experimental heat of combustion of an alcohol, the set-up was assembled as shown
below. Experimental results and standard constants were tabulated:
Thermometer
Experimental Data:
Initial mass of spirit burner with alcohol
Final mass of spirit burner with alcohol
Initial temperature of water in calorimeter before heating
Final temperature of water in calorimeter after heating
Standard Constants:
Specific heat of water
Molar mass of the alcoholused
Theoretical heat of combustion of the alcohol
224.59 grams
223.42 grams
shield
Copper calorimeter
26.3°C
Clamp
56.5 °C
(250 ml)
4.184 Joule/gram C
Spinit burner
Liquid fuel
46 gram per mole
-1368 kJoule per mole
Express how close or how far the experimental value for the heat of combustion is to the theoretical
expected value.
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning