to en 100. mL of 1.0 M HCI is added to a 2.0 g piece of CaCO3, CO2 is produced at a certain rate. Which of the changes below will NOT increase the rate of this When М НСПIS reaction? a. Adding 100. mL of 2.0 M HCl in place of 100. mL of 1.0 M HCl. b. Heating the 100. mL of 1.0 M HCl before adding it to the СаСОз. c. Adding 100. mL of 1.0 M HCl to 2.0 g of powdered CaCO3. d. Adding 150. mL of 1.0 M HCl in place of 100. mL of 1.0 M HCI.
to en 100. mL of 1.0 M HCI is added to a 2.0 g piece of CaCO3, CO2 is produced at a certain rate. Which of the changes below will NOT increase the rate of this When М НСПIS reaction? a. Adding 100. mL of 2.0 M HCl in place of 100. mL of 1.0 M HCl. b. Heating the 100. mL of 1.0 M HCl before adding it to the СаСОз. c. Adding 100. mL of 1.0 M HCl to 2.0 g of powdered CaCO3. d. Adding 150. mL of 1.0 M HCl in place of 100. mL of 1.0 M HCI.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 129MP: A gaseous material XY(g) dissociates to some extent to produce X(g) and Y(g): XY(g)X(g)+Y(g) A...
Related questions
Question
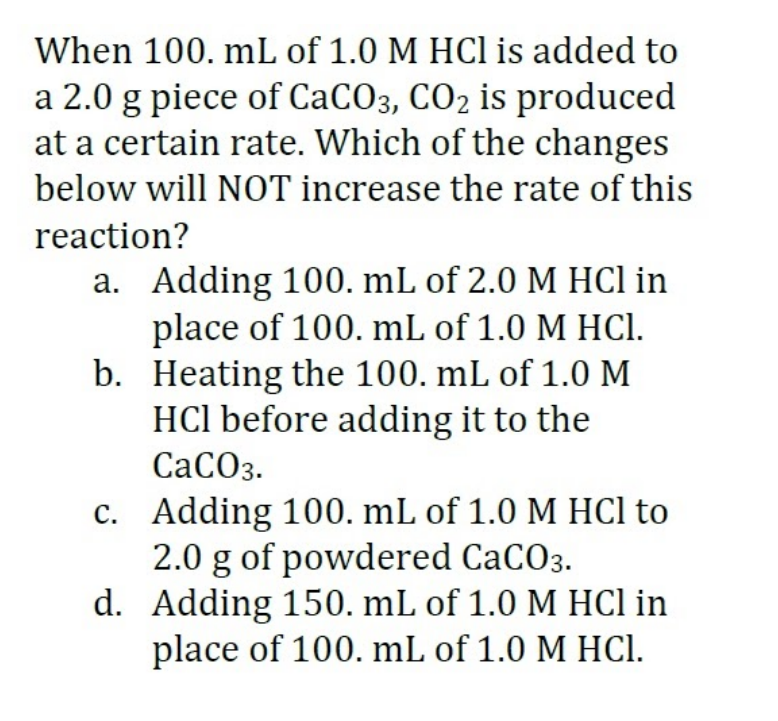
Transcribed Image Text:When 100. mL of 1.0 M HCl is added to
a 2.0 g piece of CACO3, CO2 is produced
at a certain rate. Which of the changes
below will NOT increase the rate of this
reaction?
a. Adding 100, mL of 2.0 M HCl in
place of 100. mL of 1.0 M HCI.
b. Heating the 100. mL of 1.0 M
HCl before adding it to the
СаСОз.
c. Adding 100. mL of 1.0 M HCl to
2.0 g of powdered CaCO3.
d. Adding 150. mL of 1.0 M HCl in
place of 100. mL of 1.0 M HCI.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning