Trial 1 Trial 2 Volume analyte (mL) 52.8 54.68 Volume titrant, final (mL) 14.68 14.55 Volume titrant, initial (mL) 0.68 0.51 Molarity of NaOH: 0.2203 M For Trial 2 ONLY: What is the molarity of citric acid in the Kool-Aid in Trial 2? Check your work carefully, including units. Be sure to consider the reaction between citric acid and sodium hydroxide in your calculations! Enter your answer to 4 decimals.
Trial 1 Trial 2 Volume analyte (mL) 52.8 54.68 Volume titrant, final (mL) 14.68 14.55 Volume titrant, initial (mL) 0.68 0.51 Molarity of NaOH: 0.2203 M For Trial 2 ONLY: What is the molarity of citric acid in the Kool-Aid in Trial 2? Check your work carefully, including units. Be sure to consider the reaction between citric acid and sodium hydroxide in your calculations! Enter your answer to 4 decimals.
Chapter9: Complexometric And Precipitation Titrations
Section: Chapter Questions
Problem 16P
Related questions
Question
ANSWER UP TO 4 DECIMALS
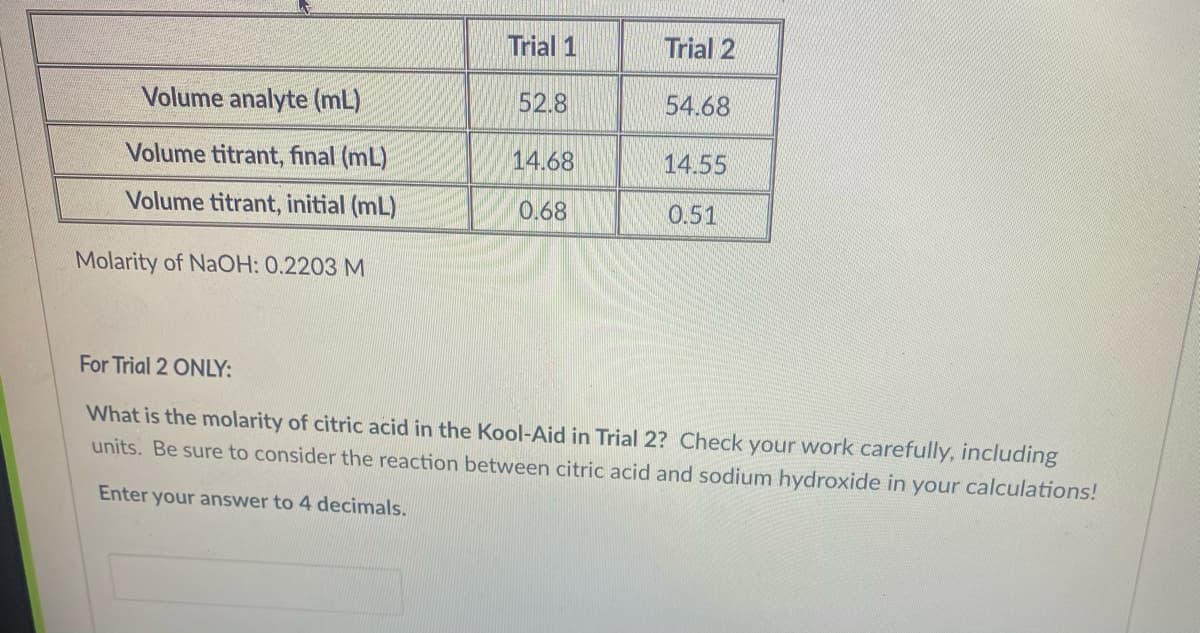
Transcribed Image Text:Trial 1
Trial 2
Volume analyte (mL)
52.8
54.68
Volume titrant, final (mL)
14.68
14.55
Volume titrant, initial (mL)
0.68
0.51
Molarity of NaOH: 0.2203 M
For Trial 2 ONLY:
What is the molarity of citric acid in the Kool-Aid in Trial 2? Check your work carefully, including
units. Be sure to consider the reaction between citric acid and sodium hydroxide in your calculations!
Enter your answer to 4 decimals.
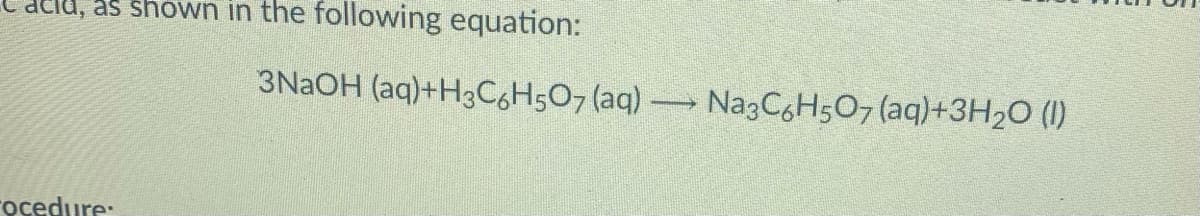
Transcribed Image Text:as shown in the following equation:
3NAOH (aq)+H3C6H5O7 (aq)
Na3CgH507 (aq)+3H2O (I)
rocedure:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



