t/takeCovalentActivity.do?locator=Dassignment-take&takeAssignmentSessionLocator%-Dassignment-take Li Be BCNO0 F Ne Na Mg 3B 4B 5B 6B 7B 8B 1B 2B AT Si PS CI Ar K Ca Sc TiVCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta WRe 0S Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf ESFM Md No Lr covalent bond(s) in order to obey the octet rule. The element oxygen would be expected to form Use the octet rule to predict the formula of the compound that would form between oxygen and chlorine , if the molecule contains only one oxygen atom and only single bonds are formed. Formula: 7 more group attempts remaining Retry Entire Group Submit Answer (Previous Email Instructor Save and E Cengage Learning | Cengage Technical Support
t/takeCovalentActivity.do?locator=Dassignment-take&takeAssignmentSessionLocator%-Dassignment-take Li Be BCNO0 F Ne Na Mg 3B 4B 5B 6B 7B 8B 1B 2B AT Si PS CI Ar K Ca Sc TiVCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta WRe 0S Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf ESFM Md No Lr covalent bond(s) in order to obey the octet rule. The element oxygen would be expected to form Use the octet rule to predict the formula of the compound that would form between oxygen and chlorine , if the molecule contains only one oxygen atom and only single bonds are formed. Formula: 7 more group attempts remaining Retry Entire Group Submit Answer (Previous Email Instructor Save and E Cengage Learning | Cengage Technical Support
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.30QAP
Related questions
Question
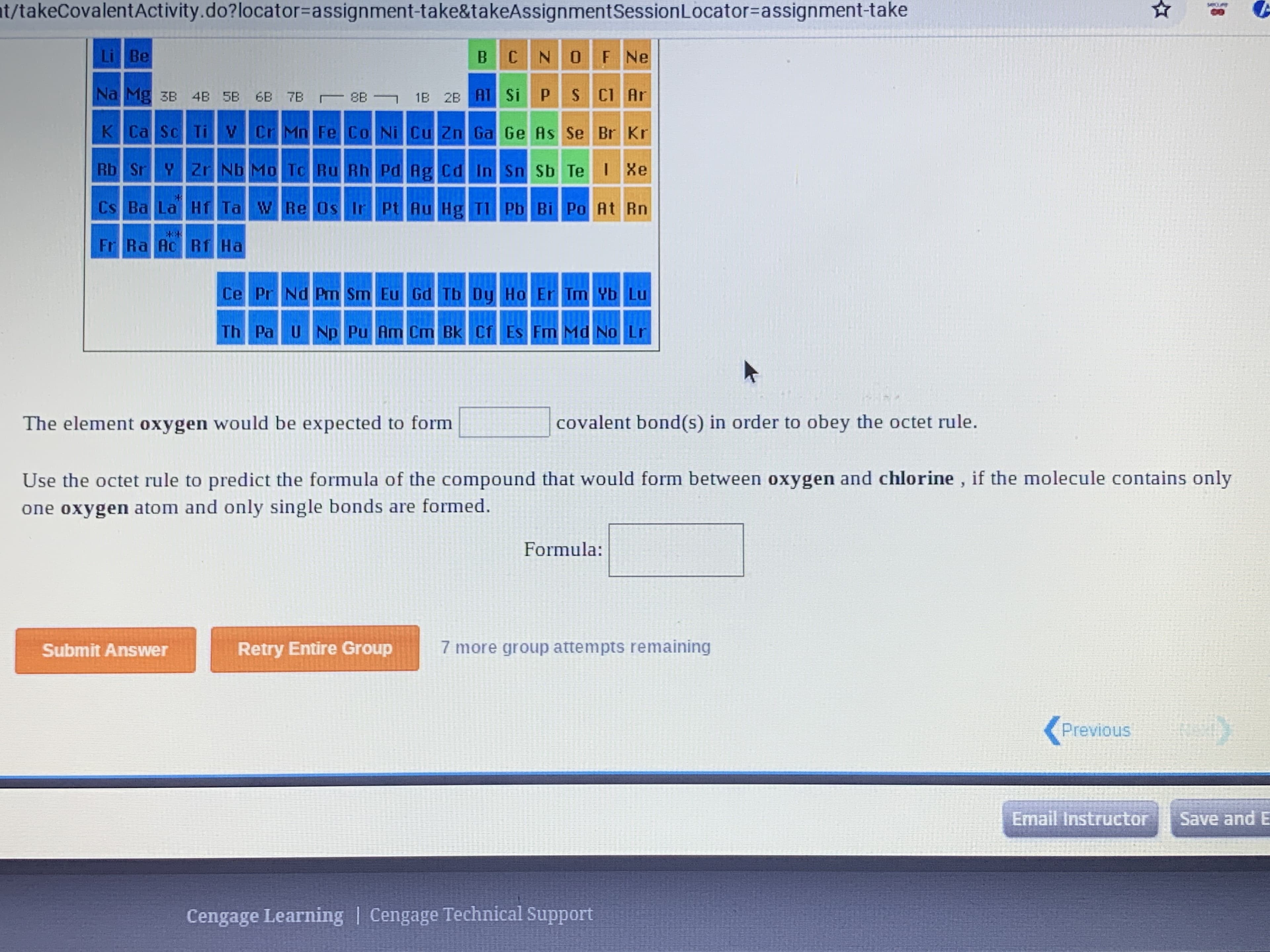
Transcribed Image Text:t/takeCovalentActivity.do?locator=Dassignment-take&takeAssignmentSessionLocator%-Dassignment-take
Li Be
BCNO0 F Ne
Na Mg 3B 4B 5B 6B 7B 8B
1B 2B AT Si PS CI Ar
K Ca Sc TiVCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Hf Ta WRe 0S Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa UNp Pu Am Cm Bk Cf ESFM Md No Lr
covalent bond(s) in order to obey the octet rule.
The element oxygen would be expected to form
Use the octet rule to predict the formula of the compound that would form between oxygen and chlorine , if the molecule contains only
one oxygen atom and only single bonds are formed.
Formula:
7 more group attempts remaining
Retry Entire Group
Submit Answer
(Previous
Email Instructor
Save and E
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning