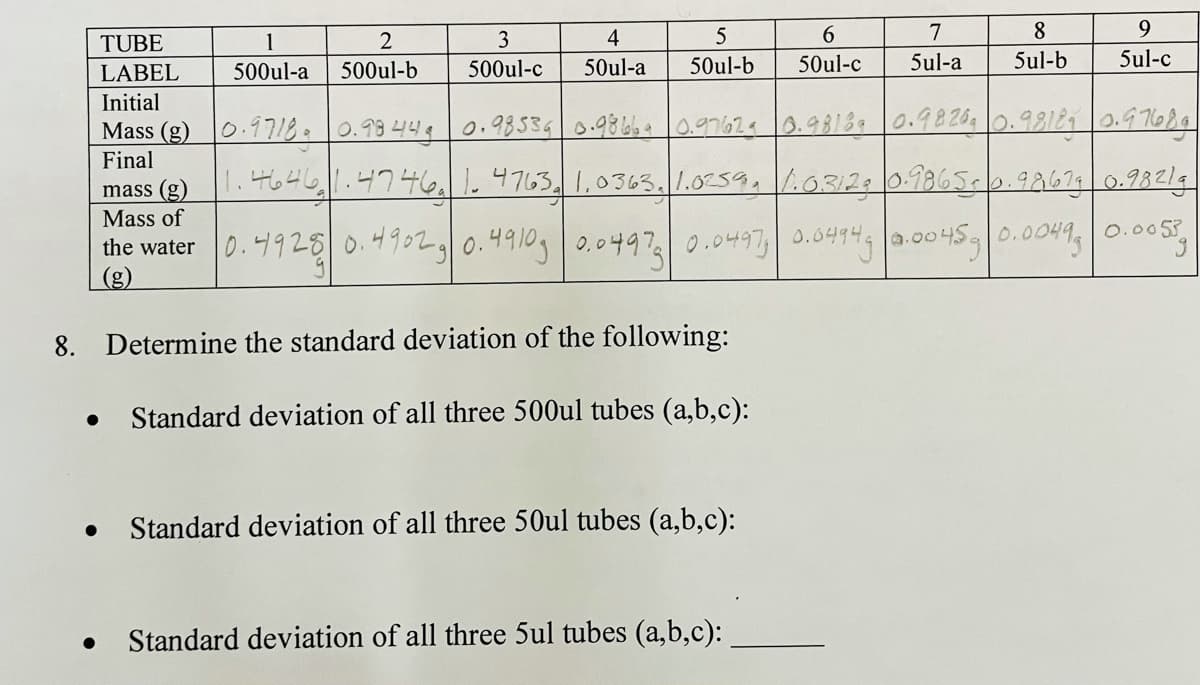
TUBE 1 2 4 6 7 8 9. 500ul-a 500ul-b 500ul-c 50ul-a 50ul-b 50ul-c 5ul-a 5ul-b 5ul-c LABEL Initial Mass (g) 0.1718= 0.99 444 0.98534 0.9866a0.9762.9818e 0.9826, 0.98189 0.9 7684 Final mass (g). 4646.47461.4763, 1,0363.1.0259.103129 0.9865-0.98673 0.98219 Mass of the water |0.니928 0.49021 0.4910, | 0.044| 0.0497| 0.04941a.00459| 0.0044, | 0.005, (g) 8. Determine the standard deviation of the following: Standard deviation of all three 500ul tubes (a,b,c): Standard deviation of all three 50ul tubes (a,b,c): Standard deviation of all three 5ul tubes (a,b,c):
TUBE 1 2 4 6 7 8 9. 500ul-a 500ul-b 500ul-c 50ul-a 50ul-b 50ul-c 5ul-a 5ul-b 5ul-c LABEL Initial Mass (g) 0.1718= 0.99 444 0.98534 0.9866a0.9762.9818e 0.9826, 0.98189 0.9 7684 Final mass (g). 4646.47461.4763, 1,0363.1.0259.103129 0.9865-0.98673 0.98219 Mass of the water |0.니928 0.49021 0.4910, | 0.044| 0.0497| 0.04941a.00459| 0.0044, | 0.005, (g) 8. Determine the standard deviation of the following: Standard deviation of all three 500ul tubes (a,b,c): Standard deviation of all three 50ul tubes (a,b,c): Standard deviation of all three 5ul tubes (a,b,c):
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.11QAP
Related questions
Question
Transcribed Image Text:1
2
3
4
6.
7
8
9.
TUBE
LABEL
500ul-a
500ul-b
500ul-c
50ul-a
50ul-b
50ul-c
5ul-a
5ul-b
5ul-c
Initial
Mass (g) 0.9718.0.99 449 0.985340.9866 0.9762 0.98189 0.9820 0.98189 0.97684
Final
mass (g).46461.47461. 4763.1,0363.1.0259,/.0312. 0.9865-0.9267 0.98219
Mass of
the water 0.4925 0.4902g0.4910g 0.0497 0.0497 0.0494s a.0045|0.0O49,|0.005
(g)
8. Determine the standard deviation of the following:
Standard deviation of all three 500ul tubes (a,b,c):
Standard deviation of all three 50ul tubes (a,b,c):
Standard deviation of all three 5ul tubes (a,b,c):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning