REPORT SHEETS DATA AND OBSERVATIONS 26.57 g 1. Mass of evaporating dish plus sample 2. Mass of evaporating dish empty dish 24.29 g 68.66 g 3. Mass of beaker plus NaCI 4. Mass of beaker 67.84 g 37.69 g 5. Mass of filter paper plus sand 6. Mass of filter paper 36.34 g 26.57-24.29 2:288 CALCULATIONS AND CONCLUSIONS 1. Calculate the mass of unknown mixture Sadium chlonide 2. Calculate the mass of NaCl recovered 68-66-67-84 0828 37.69-36-34 し35g 3. Calculate the mass of sand recovered 4. Calculate the percentage of NaCl in your unknown mixture 5. Calculate the percent sand in your unknown mixture 6. Calculate the total mass of sand and salt recovered 7. Calculate the percent recovery of the components
REPORT SHEETS DATA AND OBSERVATIONS 26.57 g 1. Mass of evaporating dish plus sample 2. Mass of evaporating dish empty dish 24.29 g 68.66 g 3. Mass of beaker plus NaCI 4. Mass of beaker 67.84 g 37.69 g 5. Mass of filter paper plus sand 6. Mass of filter paper 36.34 g 26.57-24.29 2:288 CALCULATIONS AND CONCLUSIONS 1. Calculate the mass of unknown mixture Sadium chlonide 2. Calculate the mass of NaCl recovered 68-66-67-84 0828 37.69-36-34 し35g 3. Calculate the mass of sand recovered 4. Calculate the percentage of NaCl in your unknown mixture 5. Calculate the percent sand in your unknown mixture 6. Calculate the total mass of sand and salt recovered 7. Calculate the percent recovery of the components
Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.103E
Related questions
Question
100%
Can you help me with questions 4, 5, 6, and 7 please?
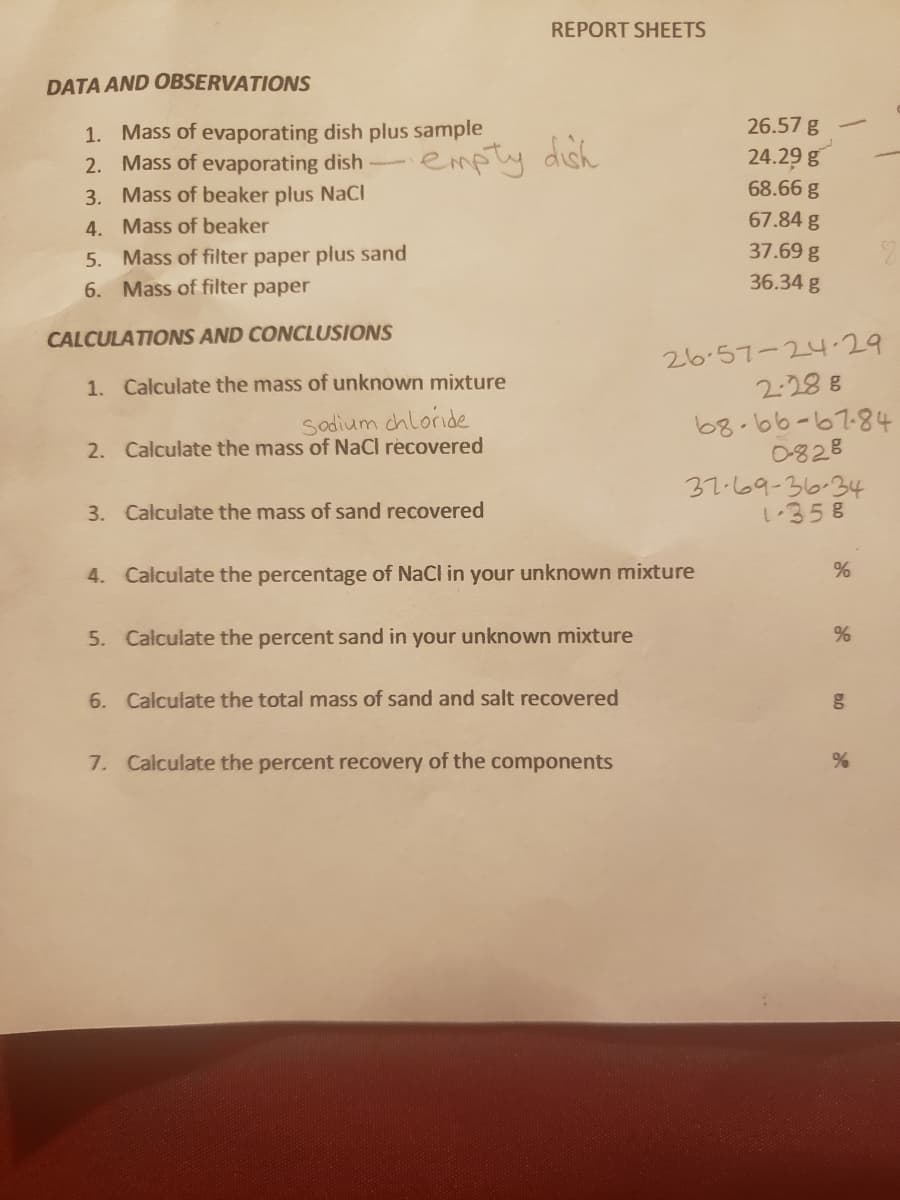
Transcribed Image Text:REPORT SHEETS
DATA AND OBSERVATIONS
26.57 g
1. Mass of evaporating dish plus sample
2. Mass of evaporating dish-
24.29 g
empty dish
68.66 g
3. Mass of beaker plus NaCI
4. Mass of beaker
67.84 g
37.69 g
5. Mass of filter paper plus sand
6. Mass of filter paper
36.34 g
CALCULATIONS AND CONCLUSIONS
26.57-24.29
2:28 8
1. Calculate the mass of unknown mixture
Sadium chloride
2. Calculate the mass of NaCl recovered
68.66-67-84
0828
37.69-36-34
し358
3. Calculate the mass of sand recovered
4. Calculate the percentage of NaCl in your unknown mixture
5. Calculate the percent sand in your unknown mixture
6. Calculate the total mass of sand and salt recovered
7. Calculate the percent recovery of the components
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning