Two objects A and B are initially at temperatures TA-350 K a two objects. If over a short interval of time Q=1 kJ is transfe a) What is the change of entropy of the hot object? ASnot 3/K Select-- v Therefore, the entropy of the hot object b) What is the change of entropy of the cold object?
Two objects A and B are initially at temperatures TA-350 K a two objects. If over a short interval of time Q=1 kJ is transfe a) What is the change of entropy of the hot object? ASnot 3/K Select-- v Therefore, the entropy of the hot object b) What is the change of entropy of the cold object?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section16.3: Measuring Dispersal Of Energy: Entropy
Problem 16.3CE
Related questions
Question
I need the answer as soon as possible
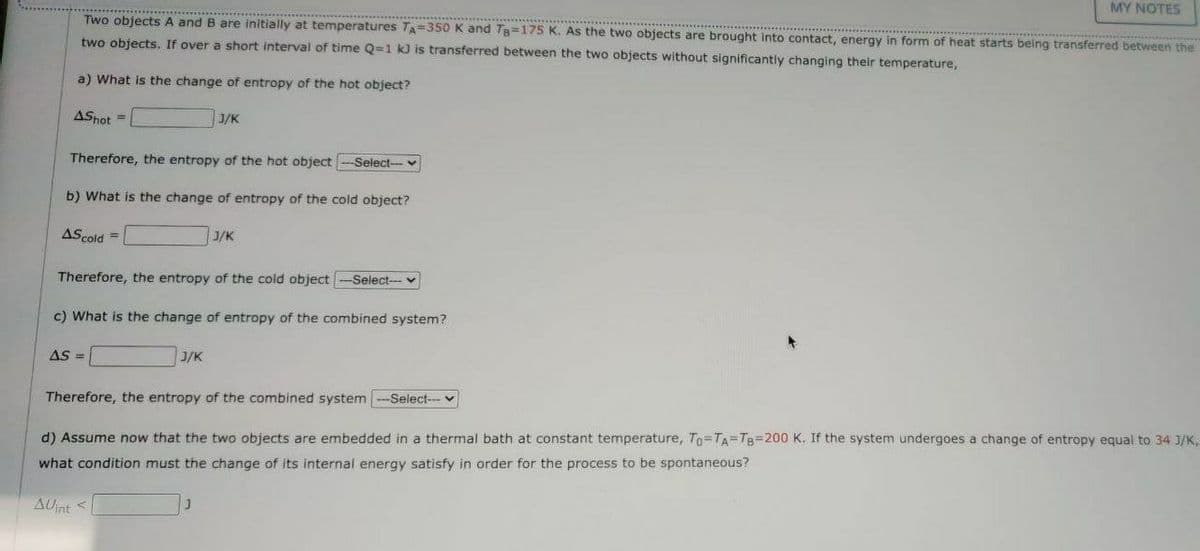
Transcribed Image Text:MY NOTES
Two objects A and B are initially at temperatures TA=350 K and Tg=175 K. As the two objects are brought into contact, energy in form of heat starts being transferred between the
two objects, If over a short interval of time Q=1 kJ is transferred between the two objects without significantly changing their temperature,
a) What is the change of entropy of the hot object?
ASnot =
J/K
Therefore, the entropy of the hot object
Select-v
b) What is the change of entropy of the cold object?
AScold =
J/K
Therefore, the entropy of the cold object
Select---
c) What is the change of entropy of the combined system?
AS =
J/K
Therefore, the entropy of the combined system
-Select-- v
d) Assume now that the two objects are embedded in a thermal bath at constant temperature, To=TA=TB=200 K. If the system undergoes a change of entropy equal to 34 3/K,
what condition must the change of its internal energy satisfy in order for the process to be spontaneous?
AUnt <
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
